
The number of $ \text{ }\sigma\text{ } $ and $ \text{ }\pi\text{ } $ in 5-oxohexanoic acid is:
Answer
555.3k+ views
Hint: The sigma bonds are formed by the head-on overlap of the atomic orbitals while the pi-orbitals are formed by the sidewise overlap of the p-atomic orbitals such that the electron cloud is above the plane of the molecule and below the plane.
Complete stepwise Solution
The structure of 5-oxohexanoic acid is as follows:
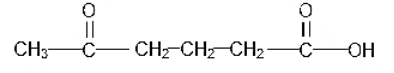
As the pi bond is formed by a sidewise overlap of the atomic orbitals and thus it is a weak bond that is present as the double bond in any molecule. As per that, there are two double bonds in the above compound and 16 single bonds. Among the two double bonds, one is the sigma bond that is formed by the head-on overlap of the $ \text{s}{{\text{p}}^{\text{2}}} $ hybrid orbitals and the other one is the pi-orbital formed by the sidewise overlap of the $ {{\text{p}}_{\text{z}}} $ orbitals.
Hence, in total there are $ \left( 16+2\text{ }=18 \right) $ sigma bonds and two pi-bonds.
Note
The sigma bonds because of the head-on combination or overlap of the atomic orbitals are stronger than the pi-bonds that are formed by the side-wise overlap of orbitals and this is because the area of overlap of the atomic or hybrid orbitals in case of the sigma bond is higher thus making it a stronger bond while the area of overlap among the atomic orbitals in smaller in case of the pi-bond and hence it is a weaker bond.
The organic compounds that possess the pi-bonds or have the double bonds are termed as “unsaturated hydrocarbons”, for example, the alkenes and the alkynes, while those that do not possess the double bonds are called the “saturated hydrocarbons”, such as the alkanes.
Complete stepwise Solution
The structure of 5-oxohexanoic acid is as follows:
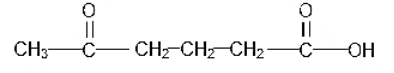
As the pi bond is formed by a sidewise overlap of the atomic orbitals and thus it is a weak bond that is present as the double bond in any molecule. As per that, there are two double bonds in the above compound and 16 single bonds. Among the two double bonds, one is the sigma bond that is formed by the head-on overlap of the $ \text{s}{{\text{p}}^{\text{2}}} $ hybrid orbitals and the other one is the pi-orbital formed by the sidewise overlap of the $ {{\text{p}}_{\text{z}}} $ orbitals.
Hence, in total there are $ \left( 16+2\text{ }=18 \right) $ sigma bonds and two pi-bonds.
Note
The sigma bonds because of the head-on combination or overlap of the atomic orbitals are stronger than the pi-bonds that are formed by the side-wise overlap of orbitals and this is because the area of overlap of the atomic or hybrid orbitals in case of the sigma bond is higher thus making it a stronger bond while the area of overlap among the atomic orbitals in smaller in case of the pi-bond and hence it is a weaker bond.
The organic compounds that possess the pi-bonds or have the double bonds are termed as “unsaturated hydrocarbons”, for example, the alkenes and the alkynes, while those that do not possess the double bonds are called the “saturated hydrocarbons”, such as the alkanes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




