
The number of $\sigma $ and $\pi $ -bonds in $F{{e}_{2}}{{(CO)}_{9}}$ , respectively are:
A. 22 $\sigma $ and 15 $\pi $
B. 23 $\sigma $ and 15 $\pi $
C. 22 $\sigma $ and 16 $\pi $
D. 15 $\sigma $ and 8 $\pi $
Answer
542.1k+ views
Hint: If p-orbitals are going to overlap axially then it leads to the formation of sigma ($\sigma $) bond and if p-orbitals are going to overlap sideways then it leads to the formation of the pi ($\pi $ ) bond. Sigma bond is stronger than pi bond.
Complete answer:
- In the question it is given to find the number of sigma bonds and number of pi bonds present in the given compound.
- The given compound is $F{{e}_{2}}{{(CO)}_{9}}$ .
- To know about how many sigma bonds and how many pi bonds present in the given compound we should know the structure of the given compound.
- The structure of the given compound is as follows.
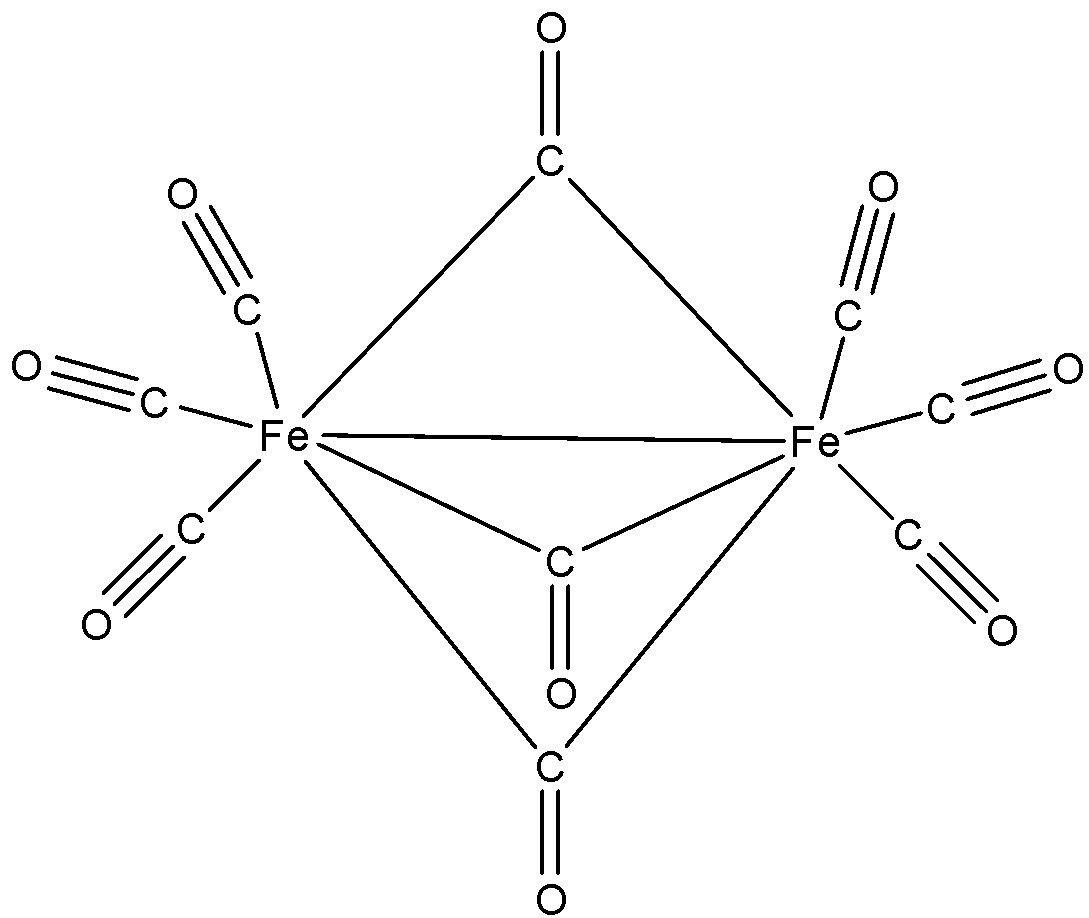
- All single bonds are sigma bonds only.
- In CO, there are three bonds. Out of those three one bond is sigma bond and the other two bonds are pi bonds.
- All Fe-CO bonds are sigma bonds in nature.
- If CO contains a double bond in the above structure then it contains one sigma bond and one pi bond in it.
- Therefore the number of sigma bonds present in the given compounds is 22 and the total number of pi bonds is 15.
So, the correct option is A.
Note:
Triple bonds are stronger than the double bond. Because in triple bonds there are two pi bonds and one pi bond while in a double bond there is one sigma bond and one pi bond. Therefore triple bonds are stronger than pi bonds.
Complete answer:
- In the question it is given to find the number of sigma bonds and number of pi bonds present in the given compound.
- The given compound is $F{{e}_{2}}{{(CO)}_{9}}$ .
- To know about how many sigma bonds and how many pi bonds present in the given compound we should know the structure of the given compound.
- The structure of the given compound is as follows.

- All single bonds are sigma bonds only.
- In CO, there are three bonds. Out of those three one bond is sigma bond and the other two bonds are pi bonds.
- All Fe-CO bonds are sigma bonds in nature.
- If CO contains a double bond in the above structure then it contains one sigma bond and one pi bond in it.
- Therefore the number of sigma bonds present in the given compounds is 22 and the total number of pi bonds is 15.
So, the correct option is A.
Note:
Triple bonds are stronger than the double bond. Because in triple bonds there are two pi bonds and one pi bond while in a double bond there is one sigma bond and one pi bond. Therefore triple bonds are stronger than pi bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




