
The number of $\sigma $ and $\pi $ bonds in ${C_2}{H_2}$ is:
(A) $0$ and $4$
(B) $2$ and $2$
(C) $3$ and $2$
(D) $4$ and $2$
Answer
540k+ views
Hint: To find the number of $\sigma $ and $\pi $ bonds in ${C_2}{H_2}$ we have to analyze the structure and hybridization of ${C_2}{H_2}$ and calculate the total number of bonds and then determine how many sigma and pi-bonds are present in the structure.
Complete step by step answer:
The chemical name of ${C_2}{H_2}$ is Ethyne. It is also called acetylene. It is an organic compound made up of carbon and hydrogen. It is the first member of the homologous series of alkynes.It is represented by the structure given below,
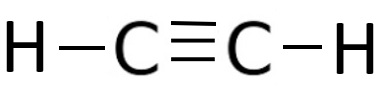
The nature of the bond found between carbon and hydrogen in ethyne is a covalent bond. The hybridization in carbon varies from $s{p^3}$ to $sp$. In case of Ethyne, the hybridization is $sp$. The geometry of the ethyne molecule is planar. In its excited state, carbon has one electron in $2s$ and three electrons in $2p$orbitals. Since two carbon atoms are present in the bonding so the one electron in $2s$orbitals forms a sigma bond with one electron in $1s$ orbital of Hydrogen and the two electrons in unhybrid $2p$ orbitals form two $\pi $ bonds with the two electrons present in$2p$orbitals of the second carbon atom. The remaining one electron forms a sigma bond with the other remaining electron. In this way, a triple bond is formed between two carbon atoms and two single bonds between carbon and hydrogen. Hence, among this one triple bond and two single bonds, the number of $\sigma $and $\pi $ bonds in ${C_2}{H_2}$ is $3$ and $2$.
So, the correct answer is Option C.
Note: Ethyne is a colorless gas. It is widely used as a fuel and a chemical building block. It is highly unstable in its pure form and thus generally handled as a solution. It is also used in various conversions in organic chemistry.
Complete step by step answer:
The chemical name of ${C_2}{H_2}$ is Ethyne. It is also called acetylene. It is an organic compound made up of carbon and hydrogen. It is the first member of the homologous series of alkynes.It is represented by the structure given below,
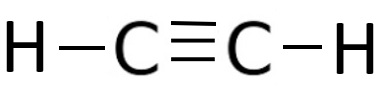
The nature of the bond found between carbon and hydrogen in ethyne is a covalent bond. The hybridization in carbon varies from $s{p^3}$ to $sp$. In case of Ethyne, the hybridization is $sp$. The geometry of the ethyne molecule is planar. In its excited state, carbon has one electron in $2s$ and three electrons in $2p$orbitals. Since two carbon atoms are present in the bonding so the one electron in $2s$orbitals forms a sigma bond with one electron in $1s$ orbital of Hydrogen and the two electrons in unhybrid $2p$ orbitals form two $\pi $ bonds with the two electrons present in$2p$orbitals of the second carbon atom. The remaining one electron forms a sigma bond with the other remaining electron. In this way, a triple bond is formed between two carbon atoms and two single bonds between carbon and hydrogen. Hence, among this one triple bond and two single bonds, the number of $\sigma $and $\pi $ bonds in ${C_2}{H_2}$ is $3$ and $2$.
So, the correct answer is Option C.
Note: Ethyne is a colorless gas. It is widely used as a fuel and a chemical building block. It is highly unstable in its pure form and thus generally handled as a solution. It is also used in various conversions in organic chemistry.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




