
The number of ${\rm{S}} = {\rm{O}}$ and ${\rm{S}} - {\rm{OH}}$ bonds present in
peroxodisulfuric acid and pyrosulfuric acid respectively are:
A) $\left( {{\rm{4}}\;{\rm{and}}\;{\rm{2}}} \right)\;{\rm{and}}\;\left( {{\rm{2}}\;{\rm{and}}\;{\rm{4}}}
\right)$
B) $\left( {2\;{\rm{and}}\;4} \right)\;{\rm{and}}\;\left( {2\;{\rm{and}}\;{\rm{4}}} \right)$
C) $\left( {4\;{\rm{and}}\;2} \right)\;{\rm{and}}\;\left( {4\;{\rm{and}}\;2} \right)$
D) $\left( {2\;{\rm{and}}\;2} \right)\;{\rm{and}}\;\left( {2\;{\rm{and}}\;2} \right)$
Answer
568.8k+ views
Hint:We know that the acid which contains oxygen in the compound, then this acid, is termed as oxyacid or oxoacid. The compound generally contains hydrogen, oxygen, and some other elements in the formula are necessary. The hydrogen bond dissociates and forms a hydrogen ion as a cation, and the oxygen bond dissociates to form an anion of the acid.
Complete step-by-step answer:As we know that, the peroxodisulfuric acid and pyrosulfuric acid are the examples of oxyacid and both contain sulfur oxygen and sulfur hydrogen bond. In the oxyacids, the oxygen is the main element.
The structure of peroxodisulfuric acid is shown below.

According to the structure of the peroxodisulfuric acid, it contains four ${\rm{S}} = {\rm{O}}$ bond and two ${\rm{S}} - {\rm{OH}}$ bond.
Similarly, the structure of pyrosulfuric acid is shown below.
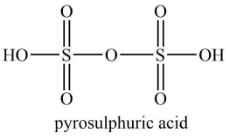
According to the structure of pyrosulfuric acid, it contains four ${\rm{S}} = {\rm{O}}$ bond and two ${\rm{S}} - {\rm{OH}}$ bond.
Therefore, the correct option for this question is C that is $\left( {4\;{\rm{and}}\;2} \right)\;{\rm{and}}\;\left( {4\;{\rm{and}}\;2} \right)$.
Note:The oxyacid is used to manufacture several dyes, explosives substances, and drugs. It is also termed as ternary compounds or acids. It is also used for the manufacture of several fertilizers. The oxyacid is used as laboratory reagent and used in the manufacture of other compounds.
Complete step-by-step answer:As we know that, the peroxodisulfuric acid and pyrosulfuric acid are the examples of oxyacid and both contain sulfur oxygen and sulfur hydrogen bond. In the oxyacids, the oxygen is the main element.
The structure of peroxodisulfuric acid is shown below.

According to the structure of the peroxodisulfuric acid, it contains four ${\rm{S}} = {\rm{O}}$ bond and two ${\rm{S}} - {\rm{OH}}$ bond.
Similarly, the structure of pyrosulfuric acid is shown below.

According to the structure of pyrosulfuric acid, it contains four ${\rm{S}} = {\rm{O}}$ bond and two ${\rm{S}} - {\rm{OH}}$ bond.
Therefore, the correct option for this question is C that is $\left( {4\;{\rm{and}}\;2} \right)\;{\rm{and}}\;\left( {4\;{\rm{and}}\;2} \right)$.
Note:The oxyacid is used to manufacture several dyes, explosives substances, and drugs. It is also termed as ternary compounds or acids. It is also used for the manufacture of several fertilizers. The oxyacid is used as laboratory reagent and used in the manufacture of other compounds.
Recently Updated Pages
Master Class 12 Business Studies: Engaging Questions & Answers for Success

Master Class 12 Biology: Engaging Questions & Answers for Success

Master Class 12 Chemistry: Engaging Questions & Answers for Success

Class 12 Question and Answer - Your Ultimate Solutions Guide

Complete reduction of benzene diazonium chloride with class 12 chemistry CBSE

How can you identify optical isomers class 12 chemistry CBSE

Trending doubts
What are the major means of transport Explain each class 12 social science CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE

India is a sovereign socialist secular democratic republic class 12 social science CBSE

How many states of matter are there in total class 12 chemistry CBSE




