
The number of isomers of ${{\rm{C}}_{\rm{5}}}{{\rm{H}}_{{\rm{10}}}}$ is?
A.10
B.11
C.12
D.13
Answer
595.2k+ views
Hint:
Isomers are compounds having the same structural formula but different arrangement of atoms in a molecule.Isomers are classified into two types -structural isomers and stereoisomers.
Complete step by step answer:
-We know that the name of the compound ${{\rm{C}}_{\rm{5}}}{{\rm{H}}_{{\rm{10}}}}$ is pentene. This compound has a total of 13 isomers. Out of these 13 isomers, 10 are structural and geometrical isomers and three of them are optical isomers.
-The structural isomers include, pent-1-ene, (E)-pent-2-ene, (Z)-pent-2-ene, 2-methylbut-1-ene, 2-methylbut-2-ene, 2-methylbut-1-ene, cyclopentane, methylcyclobutane, 1,1-dimethylcyclopropane, ethyl cyclopropane, 1,2-dimethylcyclopropane. The three optical isomers include (1R,2S)-1,2-dimethylcyclopropane, (1S,2S)-1,2- dimethyl cyclopropane, (1R,2R)-1,2-dimethylcyclopropane.
We can draw the structure of these 13 isomers in the following way.
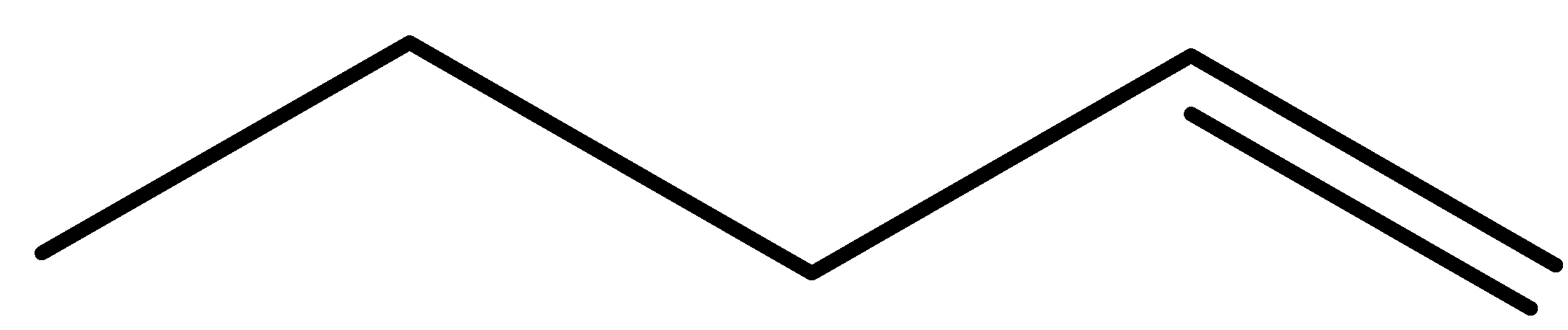
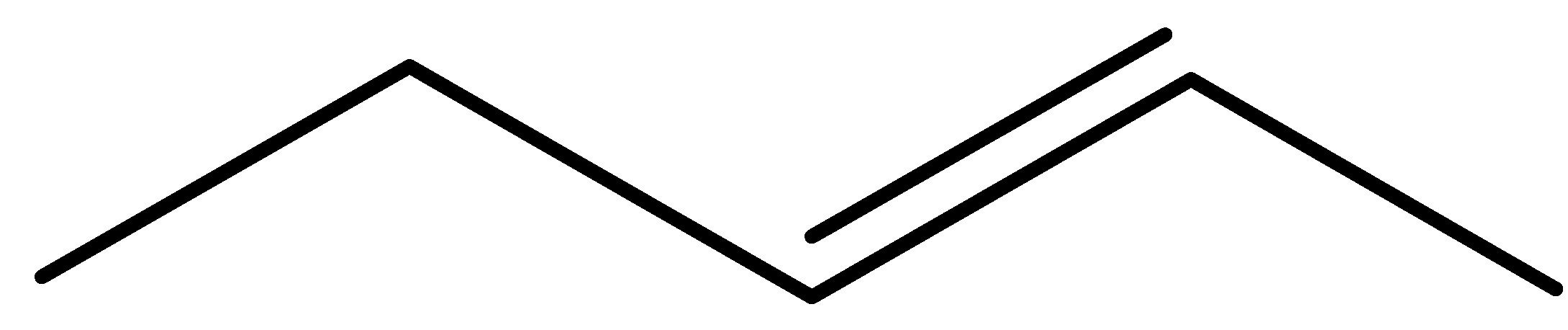
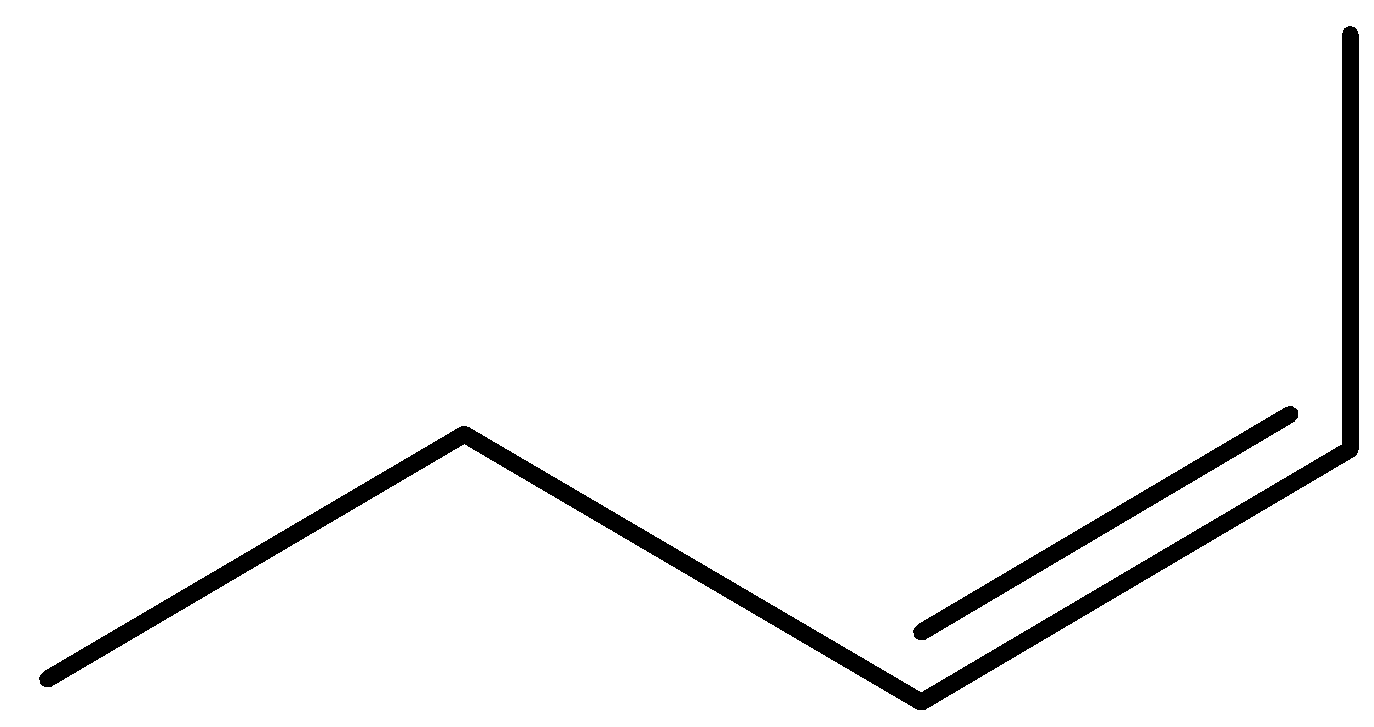
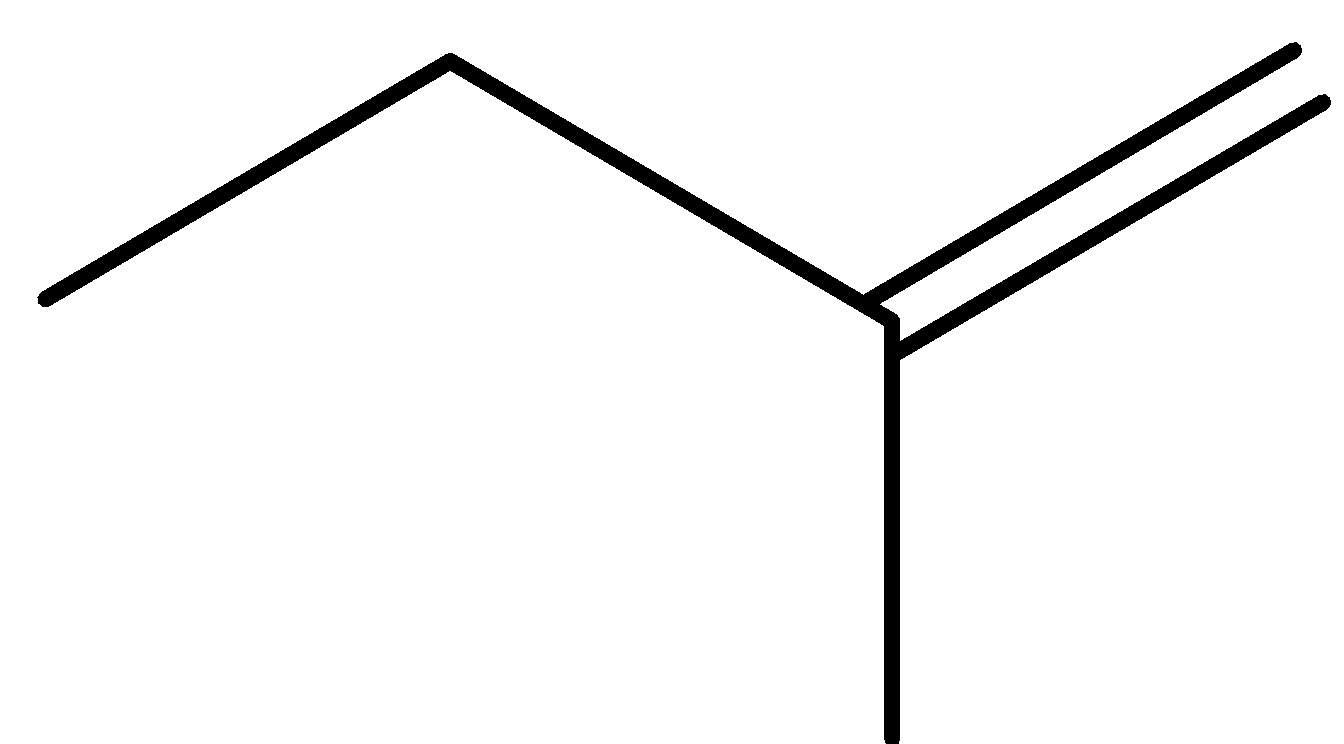
pent-1-ene (E)-pent-2-ene (Z)-pent-2-ene 2-methylbut-1-ene
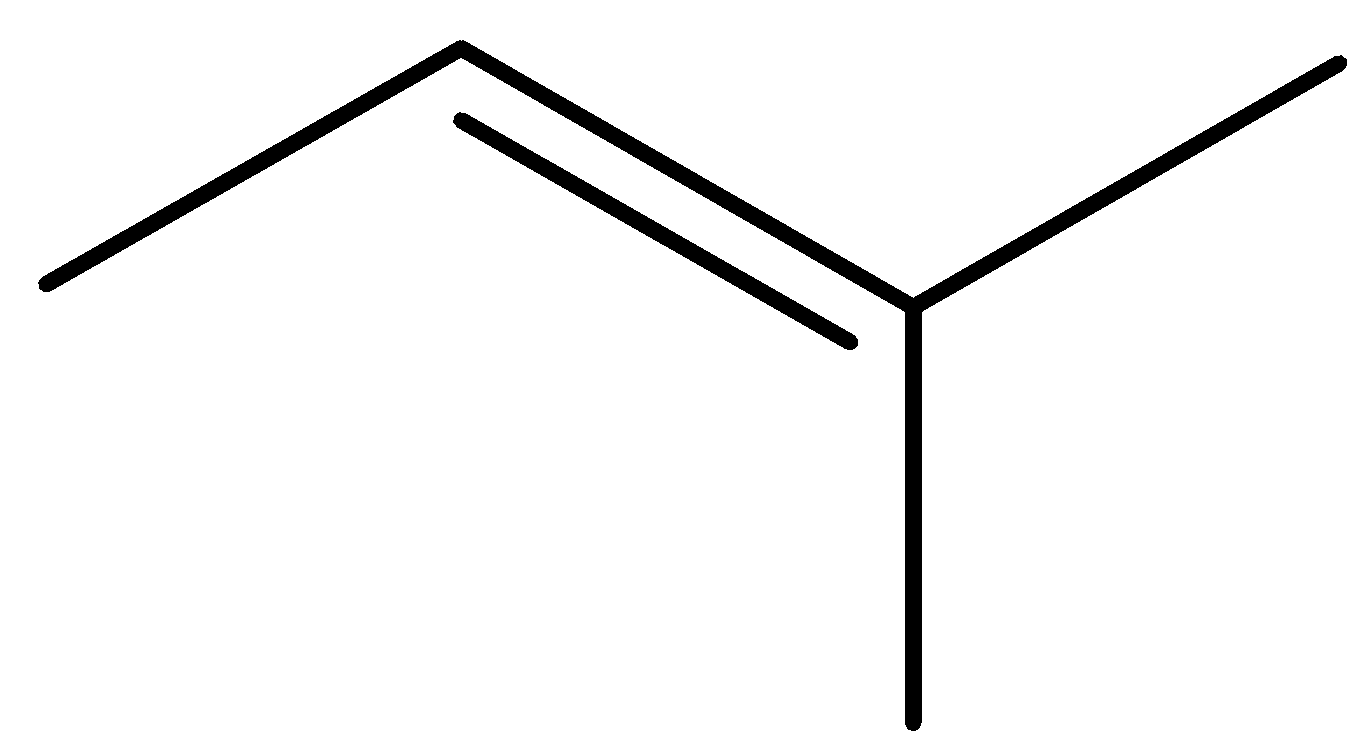
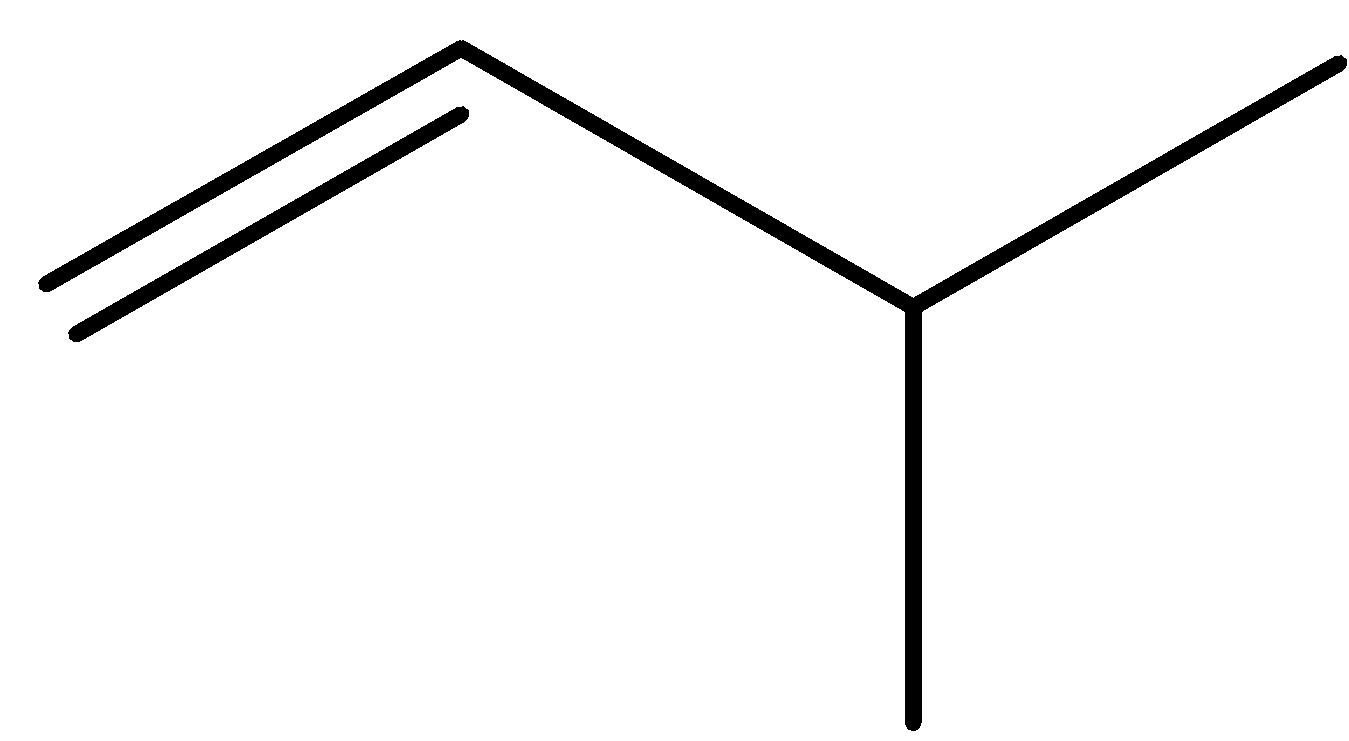
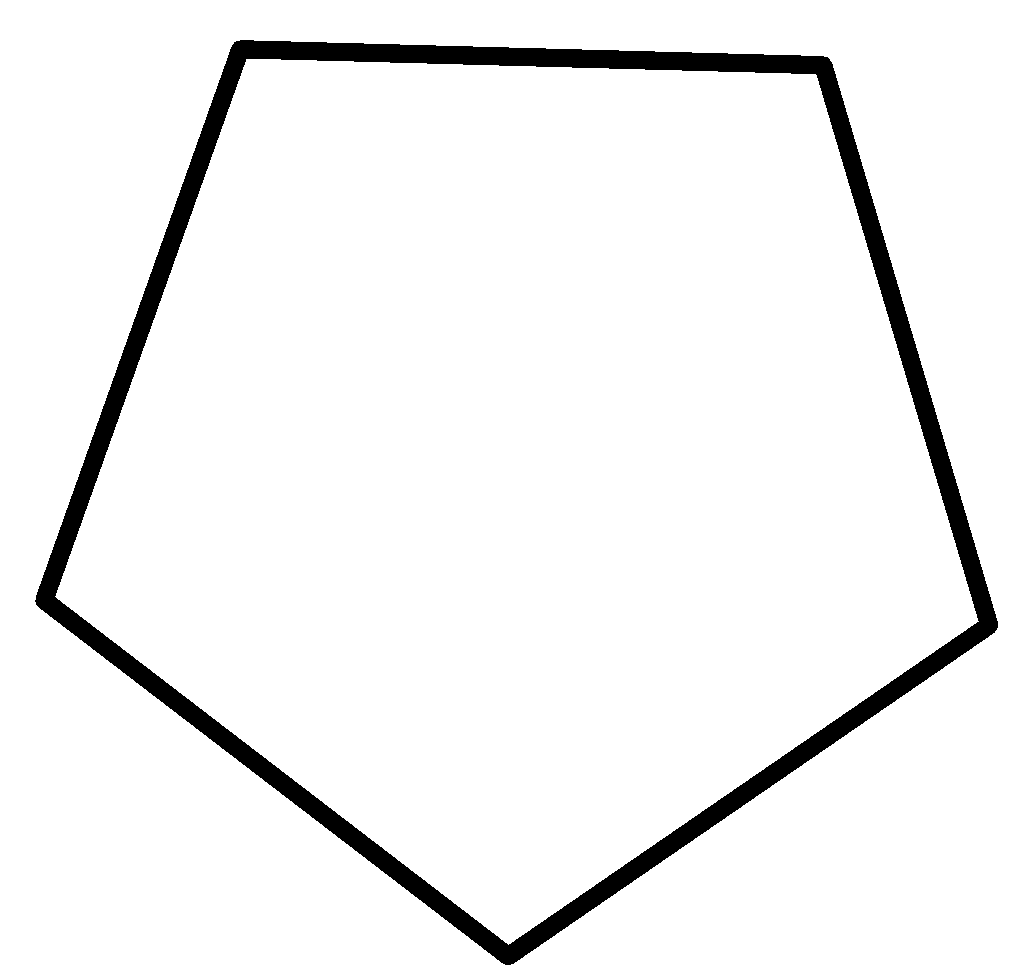
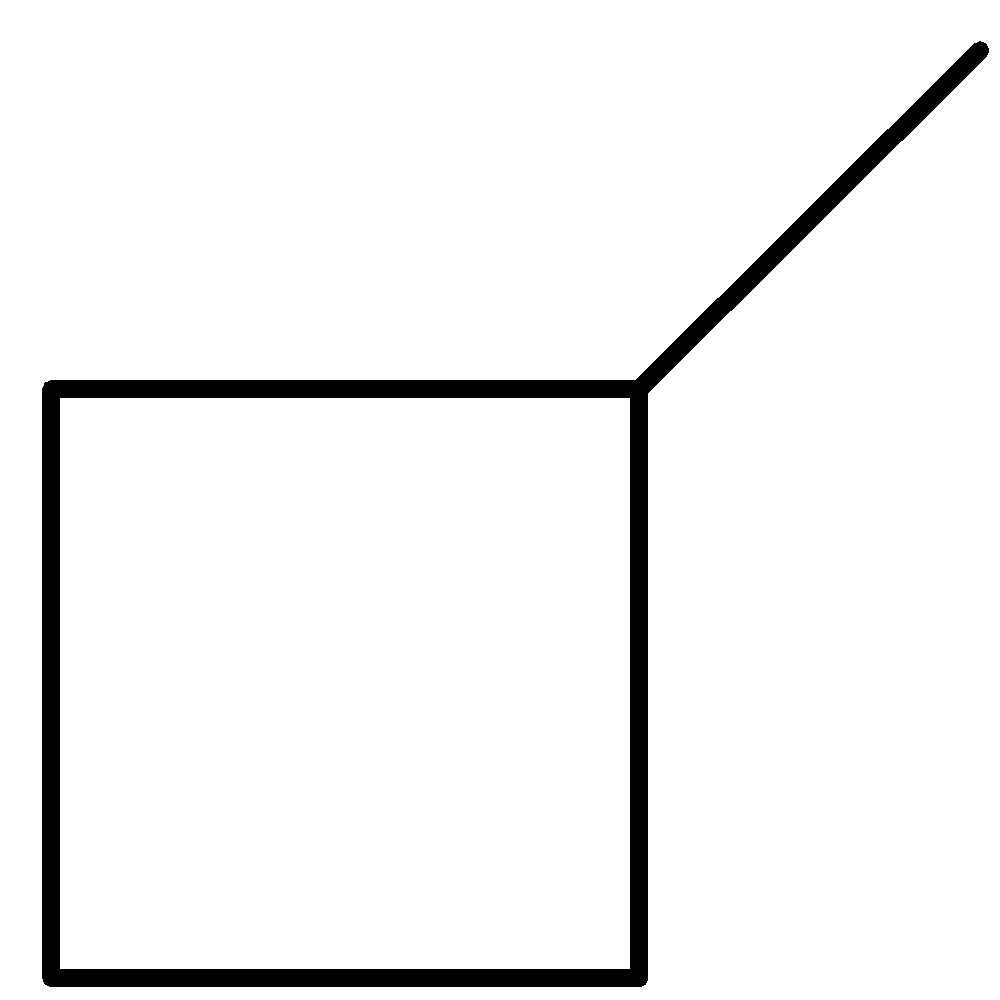
2-methylbut-2-ene 2-methylbut-1-ene cyclopentane methylcyclobutane
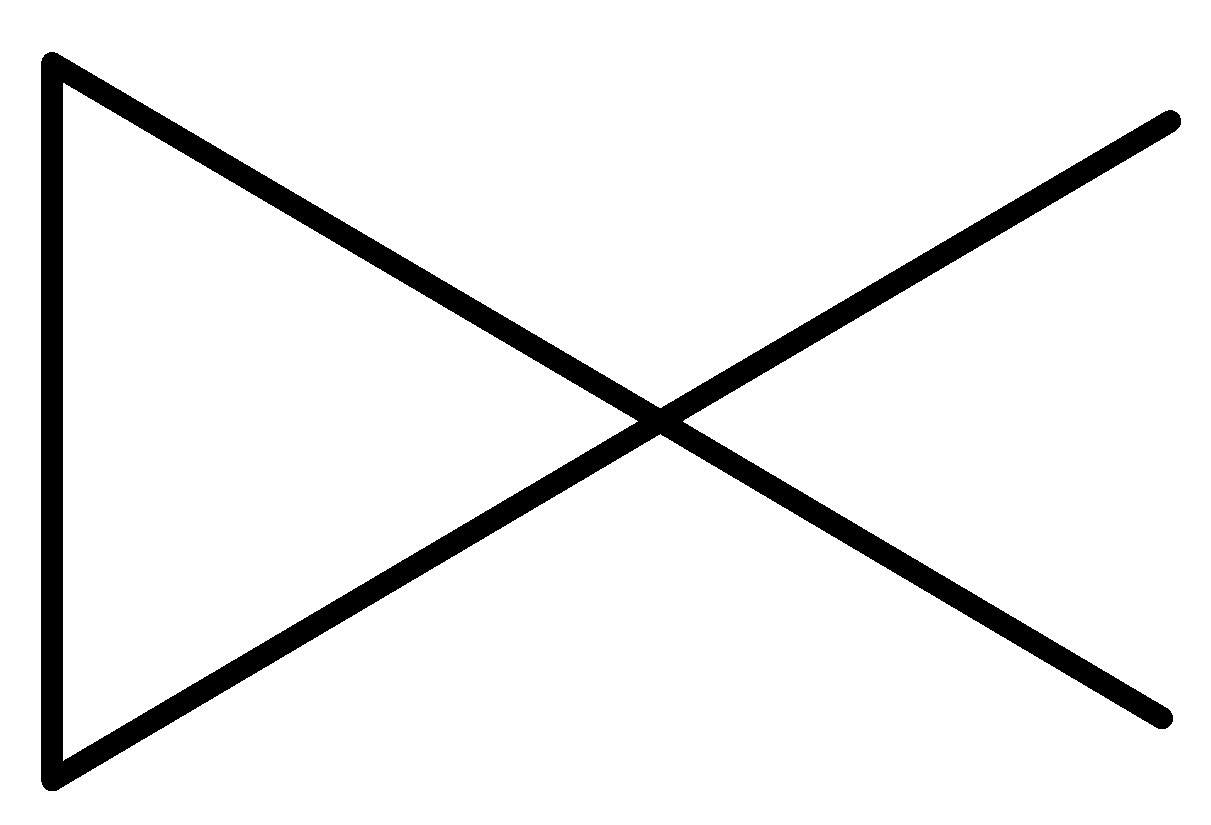
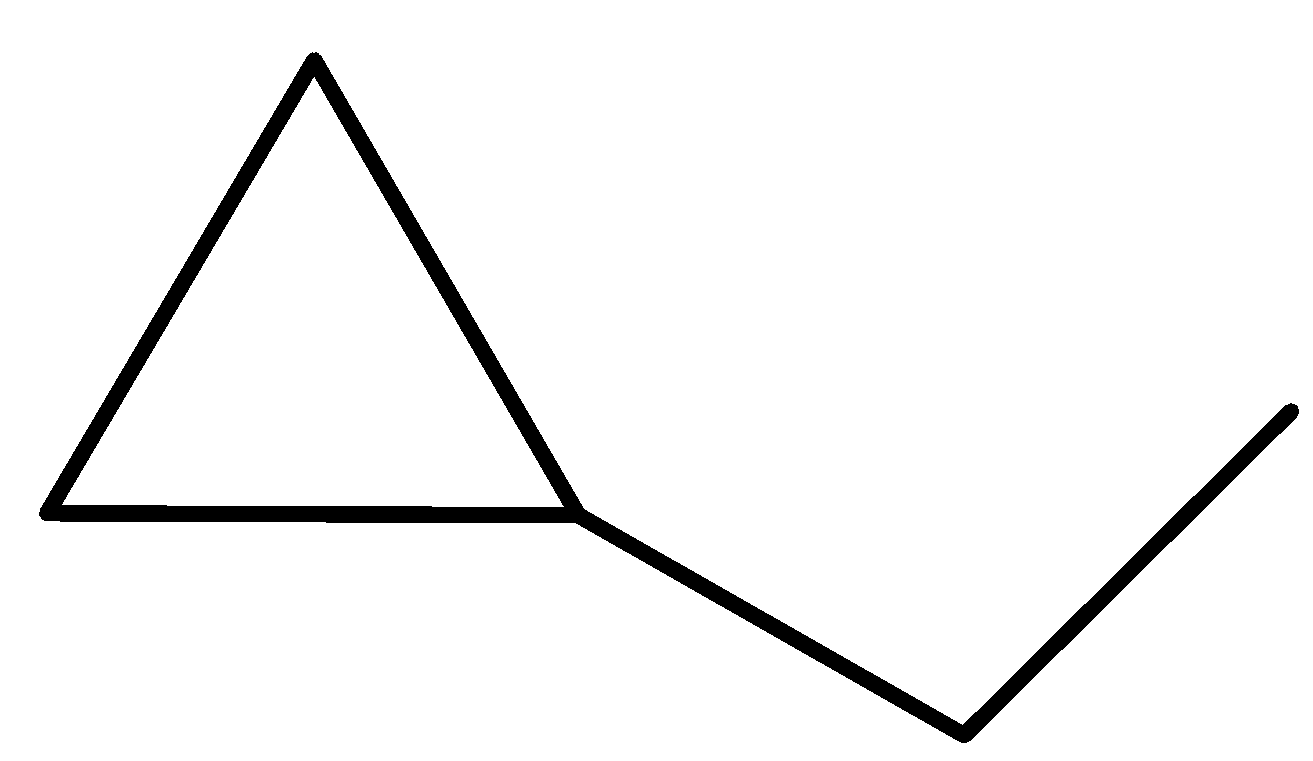
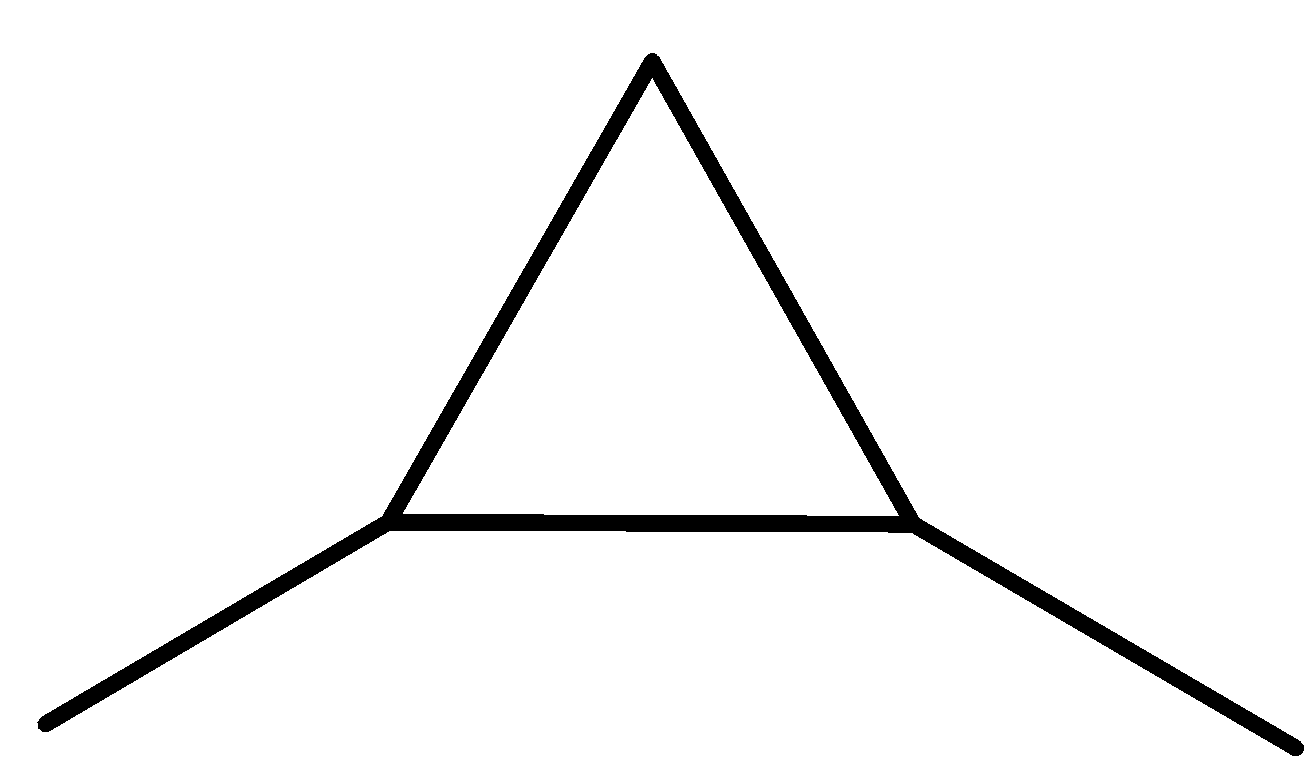
1,1-dimethylcyclopropane ethyl cyclopropane 1,2-dimethylcyclopropane
-The ten structures given above are the structural isomers of the compound ${{\rm{C}}_{\rm{5}}}{{\rm{H}}_{{\rm{10}}}}$
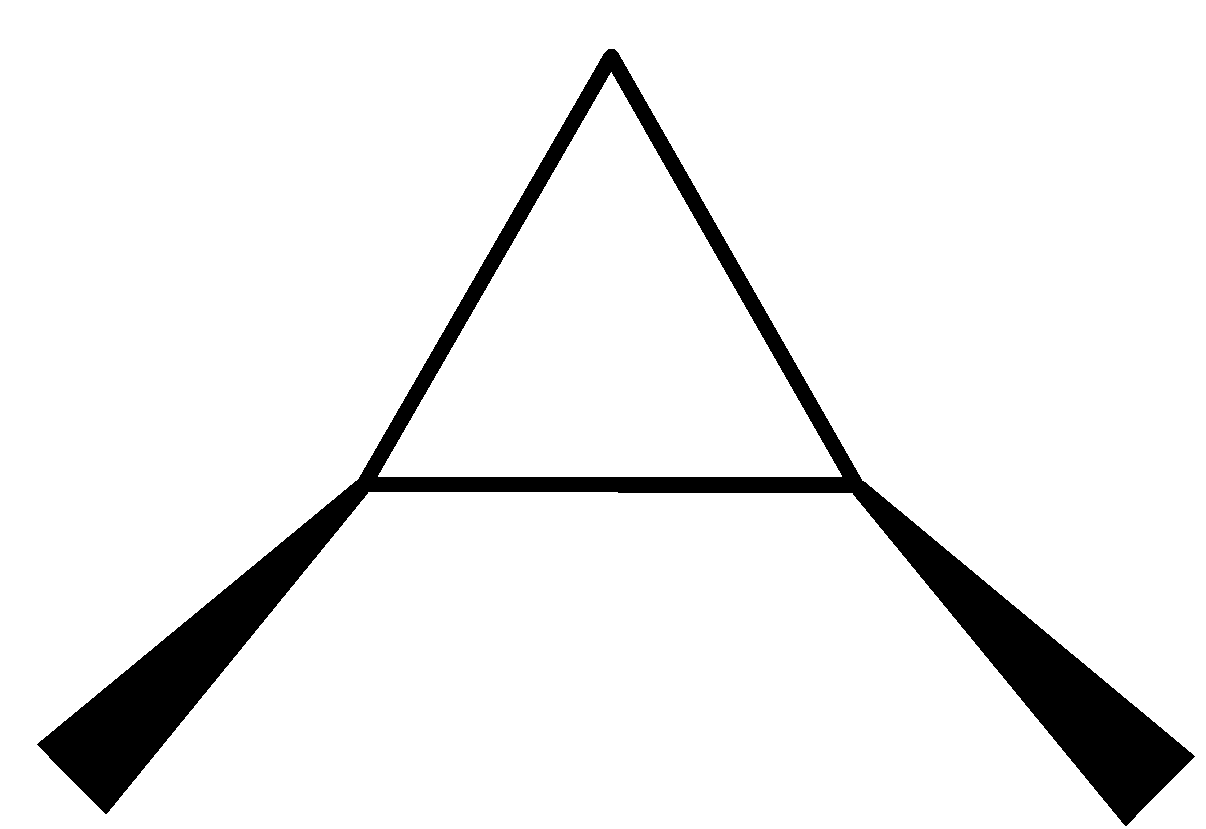
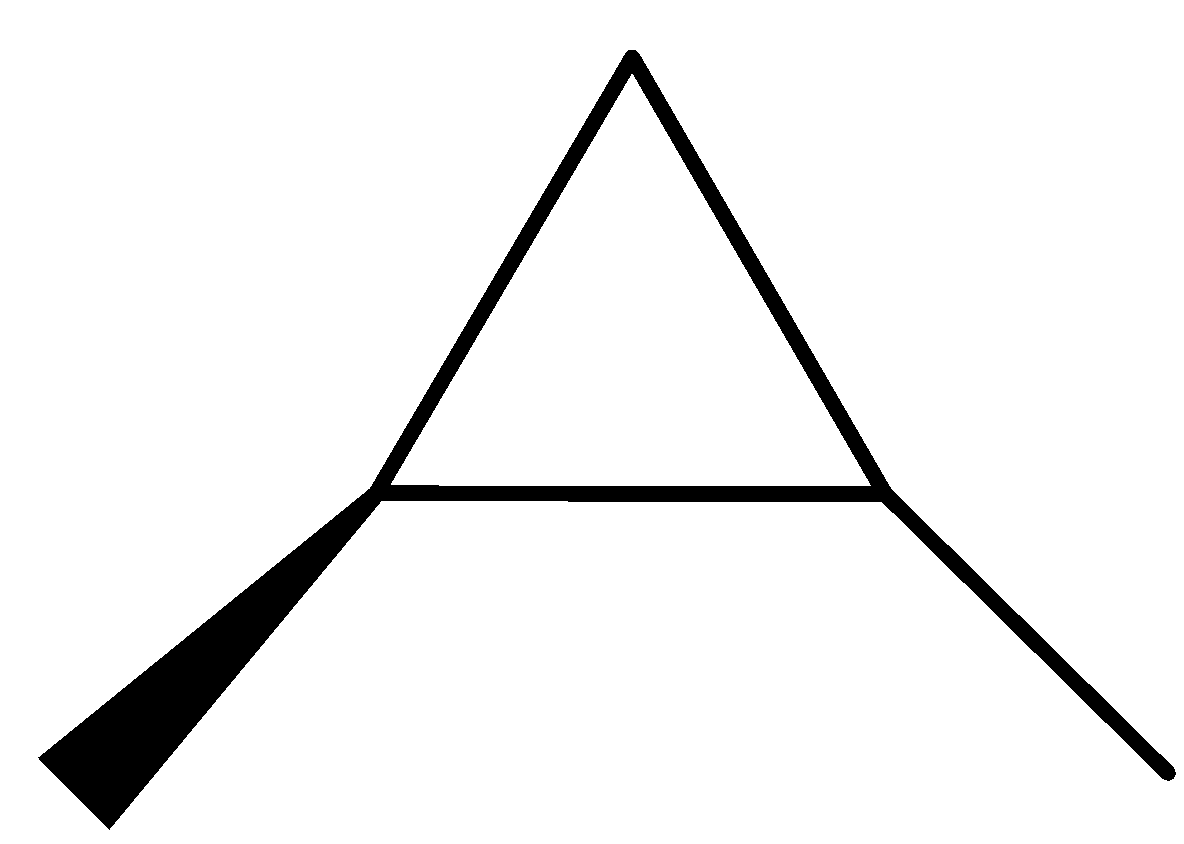
(1R,2S)-1,2-dimethylcyclopropane (1S,2S)-1,2- dimethyl cyclopropane
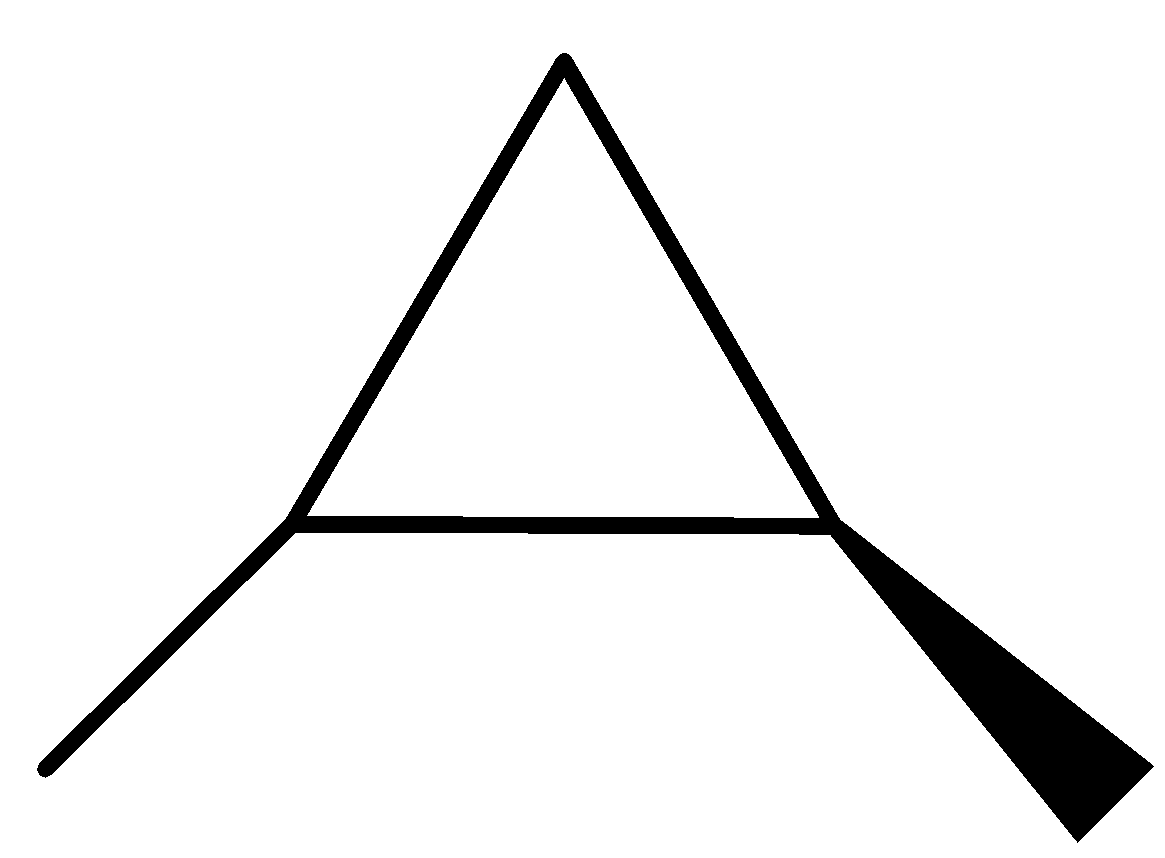
(1R,2R)-1,2-dimethylcyclopropane.
The three structures given above are the optical isomers of the compound ${{\rm{C}}_{\rm{5}}}{{\rm{H}}_{{\rm{10}}}}$.
Now, lets see that there are 13 isomers that can be drawn for the compound${{\rm{C}}_{\rm{5}}}{{\rm{H}}_{{\rm{10}}}}$,therefore, out of the given four options, D is the correct option. A, B and C are incorrect options.
Note:
Students may get confused while identifying structural and optical isomers. It is important to note that structural isomers are those isomers of a compound that has the same structural formula, but in the isomers, the arrangements of the groups in the compounds are different, while optical isomers are identified by the interaction they have with that of the plane polarized light. The optical isomers are usually non superimposable mirror images of each other.
Isomers are compounds having the same structural formula but different arrangement of atoms in a molecule.Isomers are classified into two types -structural isomers and stereoisomers.
Complete step by step answer:
-We know that the name of the compound ${{\rm{C}}_{\rm{5}}}{{\rm{H}}_{{\rm{10}}}}$ is pentene. This compound has a total of 13 isomers. Out of these 13 isomers, 10 are structural and geometrical isomers and three of them are optical isomers.
-The structural isomers include, pent-1-ene, (E)-pent-2-ene, (Z)-pent-2-ene, 2-methylbut-1-ene, 2-methylbut-2-ene, 2-methylbut-1-ene, cyclopentane, methylcyclobutane, 1,1-dimethylcyclopropane, ethyl cyclopropane, 1,2-dimethylcyclopropane. The three optical isomers include (1R,2S)-1,2-dimethylcyclopropane, (1S,2S)-1,2- dimethyl cyclopropane, (1R,2R)-1,2-dimethylcyclopropane.
We can draw the structure of these 13 isomers in the following way.




pent-1-ene (E)-pent-2-ene (Z)-pent-2-ene 2-methylbut-1-ene




2-methylbut-2-ene 2-methylbut-1-ene cyclopentane methylcyclobutane



1,1-dimethylcyclopropane ethyl cyclopropane 1,2-dimethylcyclopropane
-The ten structures given above are the structural isomers of the compound ${{\rm{C}}_{\rm{5}}}{{\rm{H}}_{{\rm{10}}}}$


(1R,2S)-1,2-dimethylcyclopropane (1S,2S)-1,2- dimethyl cyclopropane

(1R,2R)-1,2-dimethylcyclopropane.
The three structures given above are the optical isomers of the compound ${{\rm{C}}_{\rm{5}}}{{\rm{H}}_{{\rm{10}}}}$.
Now, lets see that there are 13 isomers that can be drawn for the compound${{\rm{C}}_{\rm{5}}}{{\rm{H}}_{{\rm{10}}}}$,therefore, out of the given four options, D is the correct option. A, B and C are incorrect options.
Note:
Students may get confused while identifying structural and optical isomers. It is important to note that structural isomers are those isomers of a compound that has the same structural formula, but in the isomers, the arrangements of the groups in the compounds are different, while optical isomers are identified by the interaction they have with that of the plane polarized light. The optical isomers are usually non superimposable mirror images of each other.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




