
The number of isomers for the compound with molecular formula \[{C_2}BrClFI\] is:
A.3
B.4
C.5
D.6
Answer
591.9k+ views
Hint: Isomers are molecules that have the same molecular formula but have a different arrangement of the atoms in the spatial arrangement. This does not include any different arrangements which are formed simply because of the rotation of the molecule as a whole or rotating about particular bonds.
Complete step by step answer:
-As we can observe from the question, the given compound has 2 Carbon atoms and four Halogen atoms, namely, Fluorine, Chlorine, Bromine, and Iodine.
-We know that any halogen will attach itself to only one other atom to form a stable bond between the two. This is because the Halogen atoms have electronic configuration of \[n{s^2}n{p^5}\]. This means that halogen elements can accept only one electron in a covalent compound and form only one stable bond at a time.
-On the other hand, carbon has the electronic configuration as \[[He]2{s^2}2{p^2}\]. This means that a carbon atom can stably form 4 covalent bonds, due to the vacant 4 spaces in the 2p orbital.
-From this and the given data, we can conclude that in order to form a stable isomer, there needs to be a double bond between the 2 carbon atoms and the halogens can be arranged in various permutations and combinations. Any other configuration would not be possible due to the lack of ability or capacity of the given elements to form covalent bonds.
-Hence, the isomers of the given compound \[{C_2}BrClFI\] are:
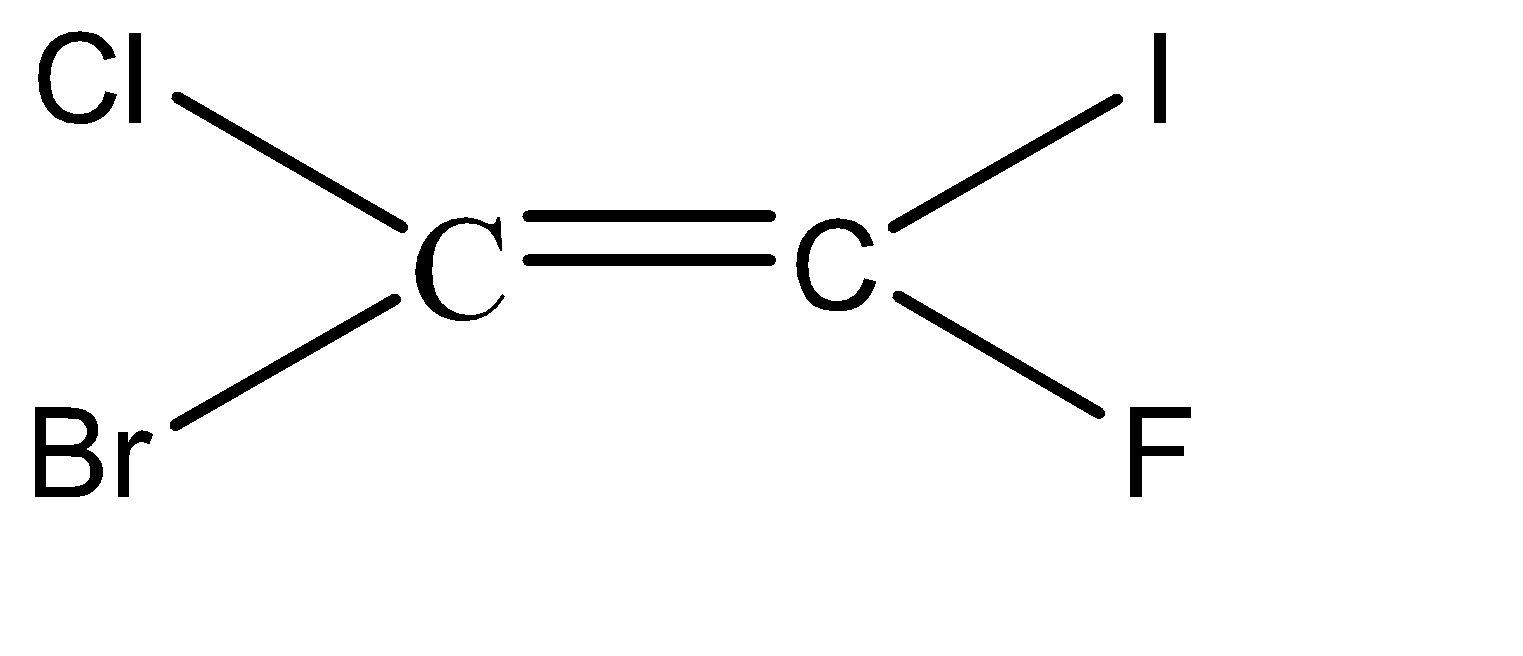

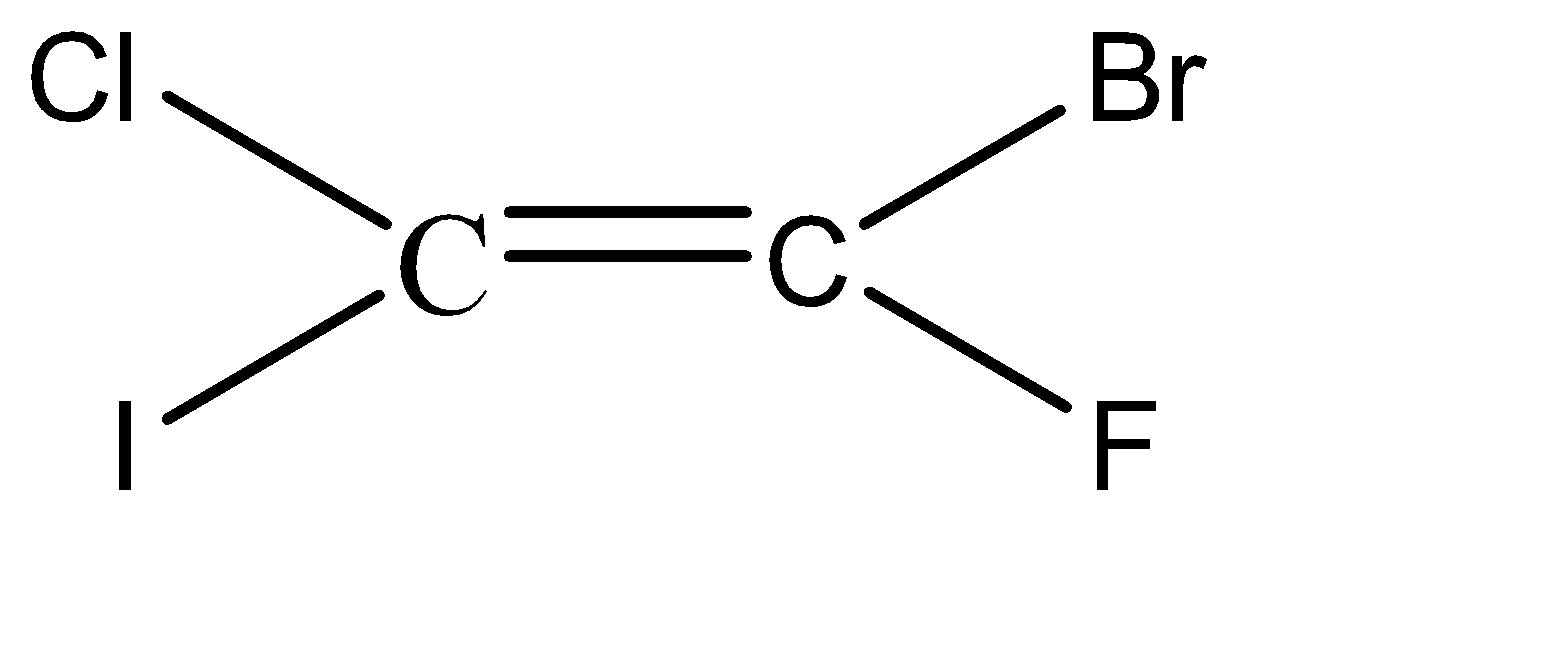

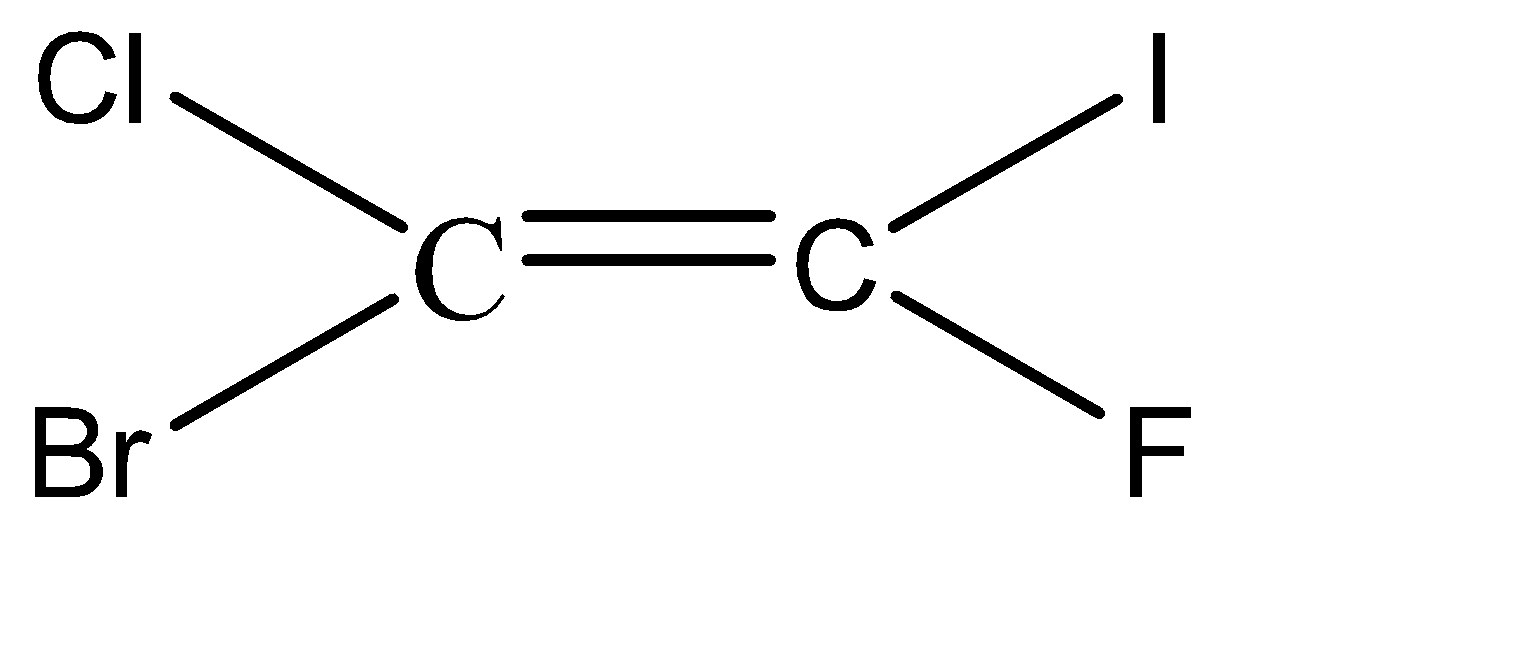
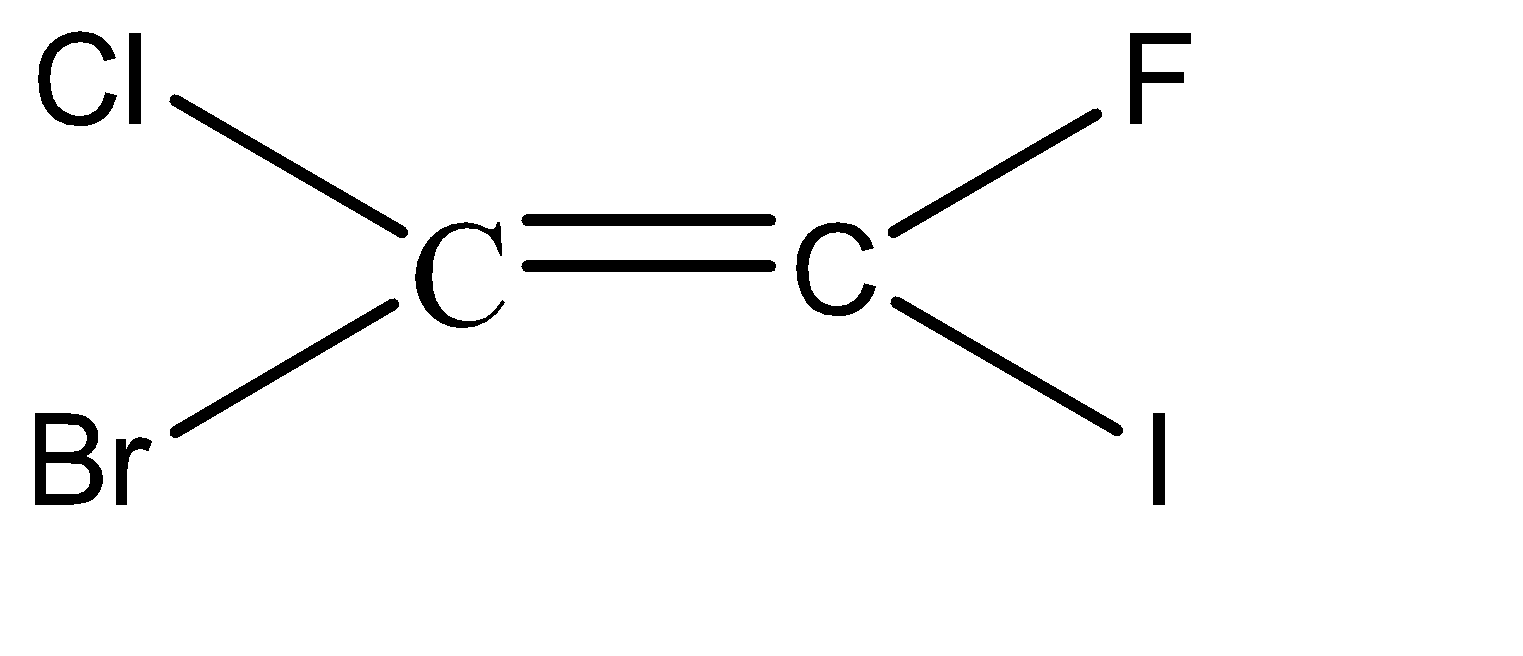
Hence, we can conclude that there are 6 isomers for the given compound \[{C_2}BrClFI\].
Hence, Option D is the correct option.
Note:
There are two main categories of isomers: stereoisomers and structural isomers. Structural isomers are isomers in which the atoms making up the molecule joined in different ways. In stereoisomers, isomers have different positions of atoms in space and are much more subtle than structural isomers.
Complete step by step answer:
-As we can observe from the question, the given compound has 2 Carbon atoms and four Halogen atoms, namely, Fluorine, Chlorine, Bromine, and Iodine.
-We know that any halogen will attach itself to only one other atom to form a stable bond between the two. This is because the Halogen atoms have electronic configuration of \[n{s^2}n{p^5}\]. This means that halogen elements can accept only one electron in a covalent compound and form only one stable bond at a time.
-On the other hand, carbon has the electronic configuration as \[[He]2{s^2}2{p^2}\]. This means that a carbon atom can stably form 4 covalent bonds, due to the vacant 4 spaces in the 2p orbital.
-From this and the given data, we can conclude that in order to form a stable isomer, there needs to be a double bond between the 2 carbon atoms and the halogens can be arranged in various permutations and combinations. Any other configuration would not be possible due to the lack of ability or capacity of the given elements to form covalent bonds.
-Hence, the isomers of the given compound \[{C_2}BrClFI\] are:






Hence, we can conclude that there are 6 isomers for the given compound \[{C_2}BrClFI\].
Hence, Option D is the correct option.
Note:
There are two main categories of isomers: stereoisomers and structural isomers. Structural isomers are isomers in which the atoms making up the molecule joined in different ways. In stereoisomers, isomers have different positions of atoms in space and are much more subtle than structural isomers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




