
The number of hydrogen atoms present in 25.6 g of sucrose \[({C_{12}}{H_{22}}{0_{11}})\] which has a molar mass of 342.3g is;
A. \[22 \times {10^{23}}\]
B. \[9.91 \times {10^{23}}\]
C. \[11 \times {10^{23}}\]
D. \[44 \times {10^{23}}\]
Answer
582.6k+ views
Hint:The common example of a disaccharide is sucrose. Sucrose contains glucose and fructose in one molecule. saccharides are a group of organic compounds. The general formula of saccharide is \[{C_n}{H_{2n}}{O_n}\] . The ratio of hydrogen and oxygen is always \[2:1\] . Saccharides are also called carbohydrates. This is biomolecules.
Complete step by step answer:
Based on the number of saccharide molecules attached saccharides are classified into four different types These types are,
Monosaccharides, disaccharides, trisaccharide, and polysaccharides, etc. sucrose is a disaccharide.
The structure of sucrose is shown below.
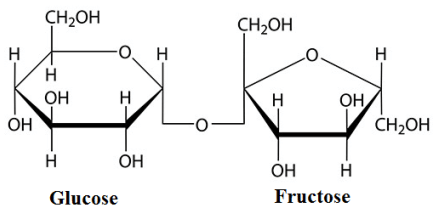
In this structure, the bonding between glucose and fructose is called glycosidic linkage. And this glycosidic linkage is at \[{C_1}\] carbon of glucose and \[{C_5}\] carbon of fructose.
Molecular mass means the weight of the 1-mole amount of a compound. And according to the mole concept, we know 1 mole of any substance is equal to its molecular weight. And 1 mole is also equal to Avogadro’s number of atoms i.e. \[6.023 \times {10^{23}}\] .
Therefore, 342.3g sucrose contains \[6.023 \times {10^{23}}\] a number of sucrose molecules.
Each sucrose has 22 numbers of hydrogen atoms. Therefore, 342.3g sucrose contains \[22 \times 6.023 \times {10^{23}}\] a number of hydrogen atoms.
Now, according to the question: The number of hydrogen atoms present in 25.6 g of sucrose \[({C_{12}}{H_{22}}{0_{11}})\] is,
\[
\dfrac{{22 \times 6.023 \times {{10}^{23}}}}{{342.3}} \times 25.6 \\
= 9.90 \times {10^{23}} \\
\]
So, the correct option is B.
Note:Remember that Sucrose contains glucose and fructose in one molecule. Based on the number of saccharide molecules attached saccharides are classified into four different types. There is another classification of saccharides based on the reducible character of the saccharide. Sucrose is a reducible saccharide.
Complete step by step answer:
Based on the number of saccharide molecules attached saccharides are classified into four different types These types are,
Monosaccharides, disaccharides, trisaccharide, and polysaccharides, etc. sucrose is a disaccharide.
The structure of sucrose is shown below.

In this structure, the bonding between glucose and fructose is called glycosidic linkage. And this glycosidic linkage is at \[{C_1}\] carbon of glucose and \[{C_5}\] carbon of fructose.
Molecular mass means the weight of the 1-mole amount of a compound. And according to the mole concept, we know 1 mole of any substance is equal to its molecular weight. And 1 mole is also equal to Avogadro’s number of atoms i.e. \[6.023 \times {10^{23}}\] .
Therefore, 342.3g sucrose contains \[6.023 \times {10^{23}}\] a number of sucrose molecules.
Each sucrose has 22 numbers of hydrogen atoms. Therefore, 342.3g sucrose contains \[22 \times 6.023 \times {10^{23}}\] a number of hydrogen atoms.
Now, according to the question: The number of hydrogen atoms present in 25.6 g of sucrose \[({C_{12}}{H_{22}}{0_{11}})\] is,
\[
\dfrac{{22 \times 6.023 \times {{10}^{23}}}}{{342.3}} \times 25.6 \\
= 9.90 \times {10^{23}} \\
\]
So, the correct option is B.
Note:Remember that Sucrose contains glucose and fructose in one molecule. Based on the number of saccharide molecules attached saccharides are classified into four different types. There is another classification of saccharides based on the reducible character of the saccharide. Sucrose is a reducible saccharide.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




