
The number of geometric isomers that can exist for square planer
\[{{[Pt(Cl)(py)(N{{H}_{3}})(N{{H}_{2}}OH)]}^{+}}\]is:
a.) 2
b.) 3
c.) 4
d.) 6
Answer
585k+ views
Hint: For finding number of geometric isomers we have to find hybridization, draw structure and see the axis of symmetry in the given complex compound.
Complete step by step solution:
We know that two or more compounds with the same formula but different arrangements of the atoms are called isomers. Many metal complexes form isomers, which are two or more compounds with the same formula but different arrangements of atoms.
Structural isomers are those which contain the same number of atoms of each kind but differ in which atoms are bonded to one another are called structural isomers, which differ in structure or bond type. For inorganic complexes, there are three types of structural isomers: ionization, coordination and linkage.
Here the coordination number of Pt is four. And the structure of \[{{[Pt(Cl)(py)(N{{H}_{3}})(N{{H}_{2}}OH)]}^{+}}\]is square planar complex. In this complex compound there is not any axis of symmetry present.
For making structural isomers we will fix one ligand and then arrange all others at different positions. Below given are the geometrical isomers of given complex compound:
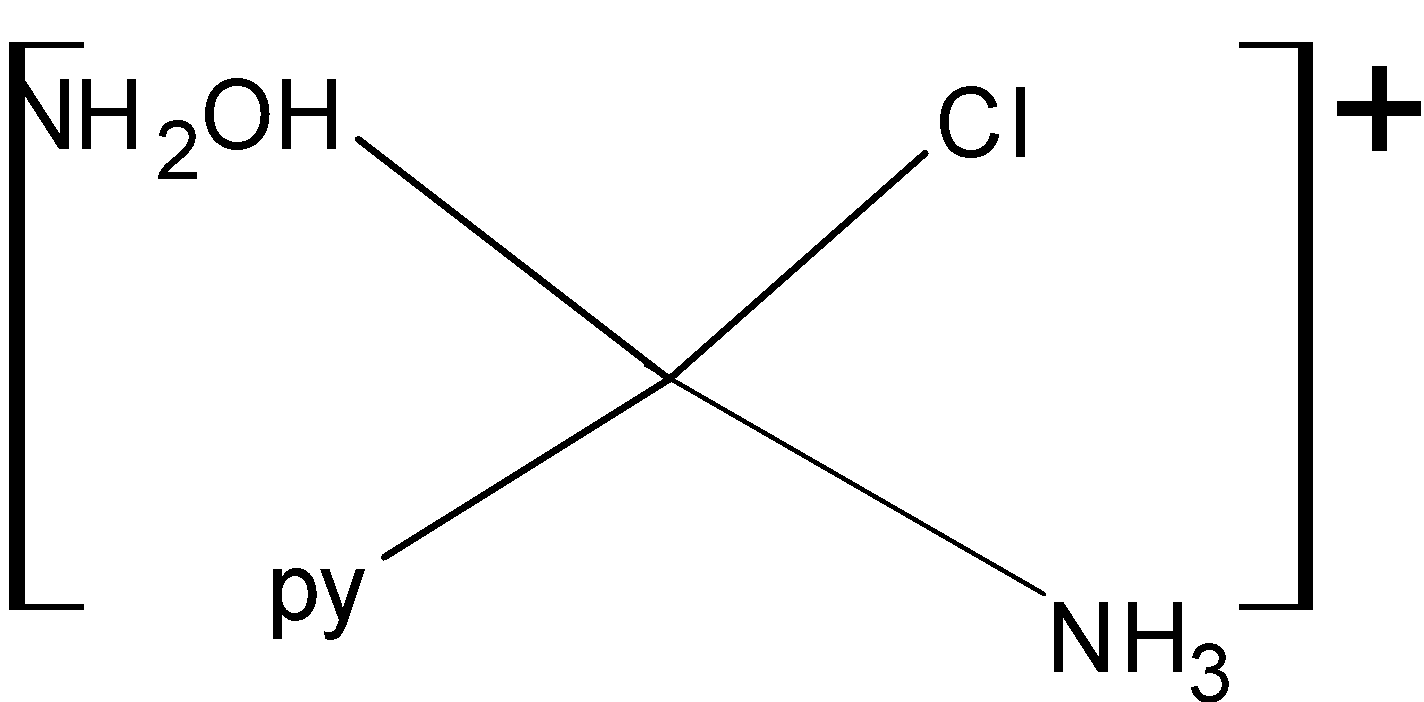
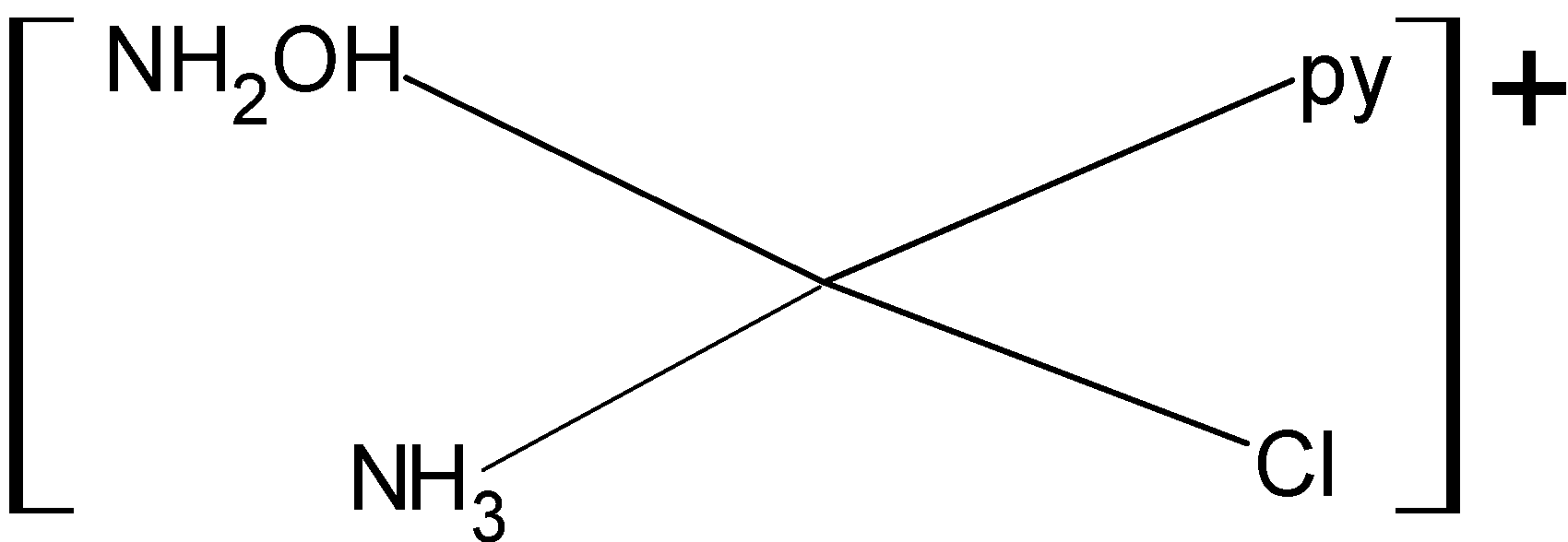
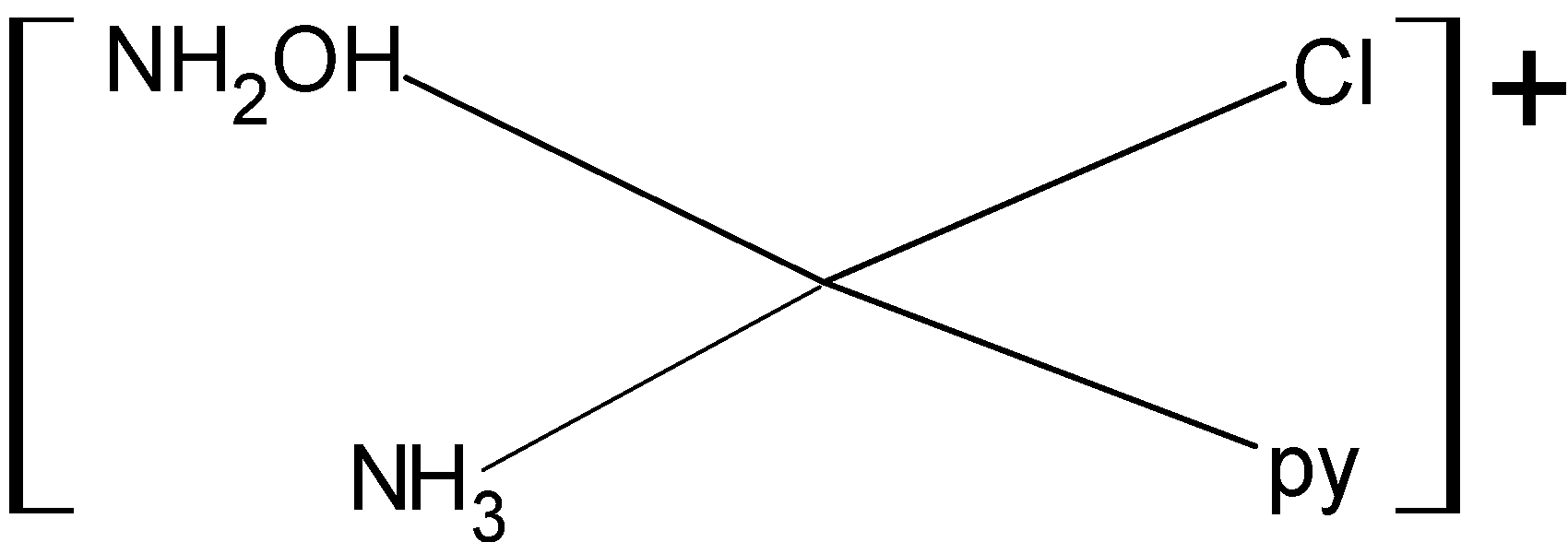
So, the given compound has three structural isomers. Then the correct answer is option “B”.
Note: First you should check the axis of symmetry in the given then start thinking about structural isomers. After drawing structural isomers once again you should confirm that all the structures are different.
Complete step by step solution:
We know that two or more compounds with the same formula but different arrangements of the atoms are called isomers. Many metal complexes form isomers, which are two or more compounds with the same formula but different arrangements of atoms.
Structural isomers are those which contain the same number of atoms of each kind but differ in which atoms are bonded to one another are called structural isomers, which differ in structure or bond type. For inorganic complexes, there are three types of structural isomers: ionization, coordination and linkage.
Here the coordination number of Pt is four. And the structure of \[{{[Pt(Cl)(py)(N{{H}_{3}})(N{{H}_{2}}OH)]}^{+}}\]is square planar complex. In this complex compound there is not any axis of symmetry present.
For making structural isomers we will fix one ligand and then arrange all others at different positions. Below given are the geometrical isomers of given complex compound:



So, the given compound has three structural isomers. Then the correct answer is option “B”.
Note: First you should check the axis of symmetry in the given then start thinking about structural isomers. After drawing structural isomers once again you should confirm that all the structures are different.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




