
The number of covalent bonds in pentane (molecular formula ${C_5}{H_{12}}$ ) is:
A. 5
B. 12
C. 17
D. 16
Answer
578.4k+ views
Hint:A covalent bond is a type of chemical bond that is formed between two atoms by the mutual sharing of electrons by the individual combining atoms. Pentane is an organic compound which has a molecular formula of ${C_5}{H_{12}}$ and is a structural framework of five carbon atoms and twelve hydrogen atoms.
Complete step by step answer:
Pentane is an organic compound with the formula ${C_5}{H_{12}}$. It belongs to the family of alkanes. It is an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them. In the IUPAC nomenclature, however, pentane means exclusively the n-pentane isomer whereas the other two isomers are called as isopentane (or simply, methylbutane) and neopentane (or simply, dimethylpropane). Cyclopentane, which is a ring structure having five carbon atoms in the closed ring, is not an isomer of pentane because it has only ten hydrogen atoms whereas pentane has twelve hydrogen atoms in its structure. The structure of n-pentane can be shown as follows:
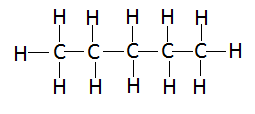
As it can be seen that the pentane has five carbon atoms and twelve hydrogen atoms and all the bonds that are visible in the diagram are covalent in nature. The number of $C - C$ bonds is equal to four and the number of $C - H$ bonds is equal to twelve. Hence, the total number of covalent bonds is equal to 16.
The correct option is D. 16.
Note:
Pentanes are components of some fuels and are employed as specialty solvents in the laboratory. Their properties are very similar to those of butanes and hexanes. Pentanes are some of the primary blowing agents used in the production of polystyrene foam and other foams.
Complete step by step answer:
Pentane is an organic compound with the formula ${C_5}{H_{12}}$. It belongs to the family of alkanes. It is an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them. In the IUPAC nomenclature, however, pentane means exclusively the n-pentane isomer whereas the other two isomers are called as isopentane (or simply, methylbutane) and neopentane (or simply, dimethylpropane). Cyclopentane, which is a ring structure having five carbon atoms in the closed ring, is not an isomer of pentane because it has only ten hydrogen atoms whereas pentane has twelve hydrogen atoms in its structure. The structure of n-pentane can be shown as follows:

As it can be seen that the pentane has five carbon atoms and twelve hydrogen atoms and all the bonds that are visible in the diagram are covalent in nature. The number of $C - C$ bonds is equal to four and the number of $C - H$ bonds is equal to twelve. Hence, the total number of covalent bonds is equal to 16.
The correct option is D. 16.
Note:
Pentanes are components of some fuels and are employed as specialty solvents in the laboratory. Their properties are very similar to those of butanes and hexanes. Pentanes are some of the primary blowing agents used in the production of polystyrene foam and other foams.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




