
The number of acyclic structural isomers represented by molecular formula \[C{}_4H{}_{10}O\] is:
A. 7
B. 6
C. 8
D. 5
Answer
582.6k+ views
Hint:A structural isomer of a compound can be understood as a compound where the isomeric compound has the same number of atoms of each element as that of the original compound, but the The molecular structure of the isomeric compound is different. To put it in simpler terms, structural isomerism can be understood as the phenomenon when the two or more compounds have the same number of atoms of each constituent element, but their molecular structures are different.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some
important basic concepts.Now, this means that the orientation of the atoms has many possible configurations. These configurations include both straight chain compounds as well as cyclic compounds. The term acyclic structural isomers refer to all the possible isomeric structures except the cyclic molecular structures.Now, these isomers can be formed by the rearrangement of the given atoms into different functional groups as well. Such isomers are identified as functional group isomers.Now, moving to the question, the compound that has been given to us has the molecular formula \[C{}_4H{}_{10}O\] . Hence, the acyclic structural isomers for this compound can be given as:
1.
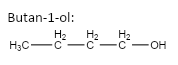
2.

3.
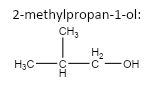
4.
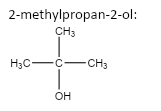
5.
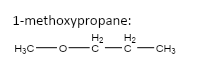
6.
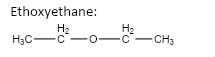
7.
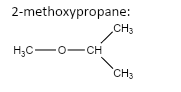
Hence, Option A is the correct option
Note: Structural isomerism is the most radical type of isomerism. It is opposed to stereoisomerism,in which the atoms and bonding scheme are the same, but only the relative spatial arrangement of the atoms are different.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some
important basic concepts.Now, this means that the orientation of the atoms has many possible configurations. These configurations include both straight chain compounds as well as cyclic compounds. The term acyclic structural isomers refer to all the possible isomeric structures except the cyclic molecular structures.Now, these isomers can be formed by the rearrangement of the given atoms into different functional groups as well. Such isomers are identified as functional group isomers.Now, moving to the question, the compound that has been given to us has the molecular formula \[C{}_4H{}_{10}O\] . Hence, the acyclic structural isomers for this compound can be given as:
1.

2.

3.

4.

5.
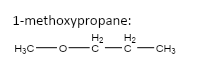
6.

7.

Hence, Option A is the correct option
Note: Structural isomerism is the most radical type of isomerism. It is opposed to stereoisomerism,in which the atoms and bonding scheme are the same, but only the relative spatial arrangement of the atoms are different.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




