
The number of 2-center-2-electron and 3-centre-2- electron bonds in ${B_2}{H_6}$ respectively are:
A) 2 and 4
B) 2 and 1
C) 2 and 2
D) 4 and 2
Answer
548.4k+ views
Hint:
To answer this question, you should recall the structure of ${B_2}{H_6}$. You should know about the properties of the banana bond and how it is different from a normal covalent bond.
Complete step by step solution:
The name Diborane suggests that it is a chemical compound that consists of boron and hydrogen atoms and has a molecular formula ${B_2}{H_6}$. It is unstable at the room temperature with a sweet odour. The molecular structure consists of four hydrogen atoms and that of two boron atoms coming on the same plane. In between these planes of the molecule, there are two dividing atoms of hydrogen.
The boron atom is known to be \[s{p^3}\] hybridized and has four hybrid orbitals. Three of these four orbitals have one electron each, and of which one is an empty orbital. The two electrons of the hybrid orbitals in each of the boron atoms form 2 bonds with the \[1s\] hydrogen atoms. The two atoms of boron left with that of each unpaired electron orbital and empty orbital forms the two bridging \[\left( {B-H-B} \right)\] bonds with that of the two 1s hydrogen atoms are also called as the banana bond. The structure can be visualised as:
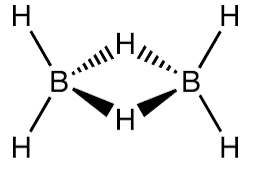
According to structure, there are four 2−centre−2−electron bonds and two 3−centre−2−electron bonds in \[{B_2}{H_6}.\]
Hence, the correct option is D.
Note:
You should know about Uses and Applications Of Diborane: Diborane has many numbers of applications in various fields, of which some are given below:
Used as a rocket propellant.
The manufacturing industry of boro-phosphosilicate which is a form of glass.
In most of the chemical reactions, it is employed as a reducing agent.
Catalyst promoter and rubber vulcanization in the polymerization reactions.
It is even used as a doping agent in the manufacturing of semiconductor devices.
To answer this question, you should recall the structure of ${B_2}{H_6}$. You should know about the properties of the banana bond and how it is different from a normal covalent bond.
Complete step by step solution:
The name Diborane suggests that it is a chemical compound that consists of boron and hydrogen atoms and has a molecular formula ${B_2}{H_6}$. It is unstable at the room temperature with a sweet odour. The molecular structure consists of four hydrogen atoms and that of two boron atoms coming on the same plane. In between these planes of the molecule, there are two dividing atoms of hydrogen.
The boron atom is known to be \[s{p^3}\] hybridized and has four hybrid orbitals. Three of these four orbitals have one electron each, and of which one is an empty orbital. The two electrons of the hybrid orbitals in each of the boron atoms form 2 bonds with the \[1s\] hydrogen atoms. The two atoms of boron left with that of each unpaired electron orbital and empty orbital forms the two bridging \[\left( {B-H-B} \right)\] bonds with that of the two 1s hydrogen atoms are also called as the banana bond. The structure can be visualised as:

According to structure, there are four 2−centre−2−electron bonds and two 3−centre−2−electron bonds in \[{B_2}{H_6}.\]
Hence, the correct option is D.
Note:
You should know about Uses and Applications Of Diborane: Diborane has many numbers of applications in various fields, of which some are given below:
Used as a rocket propellant.
The manufacturing industry of boro-phosphosilicate which is a form of glass.
In most of the chemical reactions, it is employed as a reducing agent.
Catalyst promoter and rubber vulcanization in the polymerization reactions.
It is even used as a doping agent in the manufacturing of semiconductor devices.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




