
The no. of hydroxyl group in pyrophosphoric acid is:
(A) 3
(B) 4
(C) 5
(D) 7
Answer
566.7k+ views
Hint: Pyrophosphoric acid is also known as di phosphoric acid. In chemical compounds, various substituents groups attach to a certain compound to form a different compound. This different compound formed from the substitution or addition of another group has properties different from the original compound. The hydroxyl group is represented as $ - OH$ .
Complete step by step answer:
Pyro phosphoric acid is the inorganic compound with the formula ${H_4}{P_2}{O_7}$ . The compound is colorless and odorless and is soluble in water.
The compound is not particularly useful, except that it has the component related to the polyphosphoric acid and pyrosulphates.
Various groups attach to the compound and provide certain properties to them. One of the groups being the hydroxyl group. This group is composed of oxygen and hydrogen and can be represented as $ - OH$
Hydroxyl groups are found at the polar heads of many neutral surfactants and form the polar part of the surfactant.
This group is also contained in many compounds of carboxylic acid, alcohol, and water.
This group usually grants the property of hydrogen bonding to the group, which in turn provides properties as higher boiling point and melting points.
The structure of pyrophosphoric acid can be represented as:
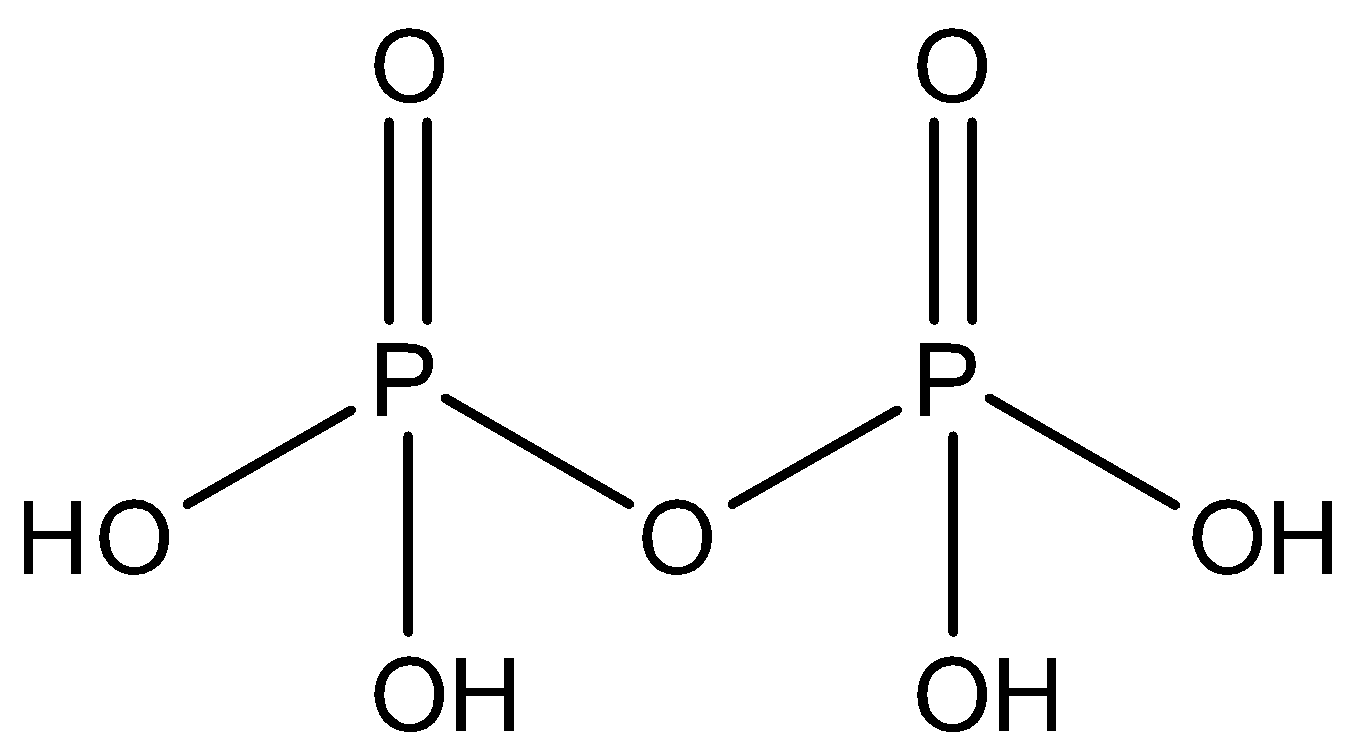
From the given figure it is clear that the number of hydroxyl groups in pyrophosphoric acid is 4
So, the correct answer is Option A .
Note: The structure of the hydroxy group is represented below
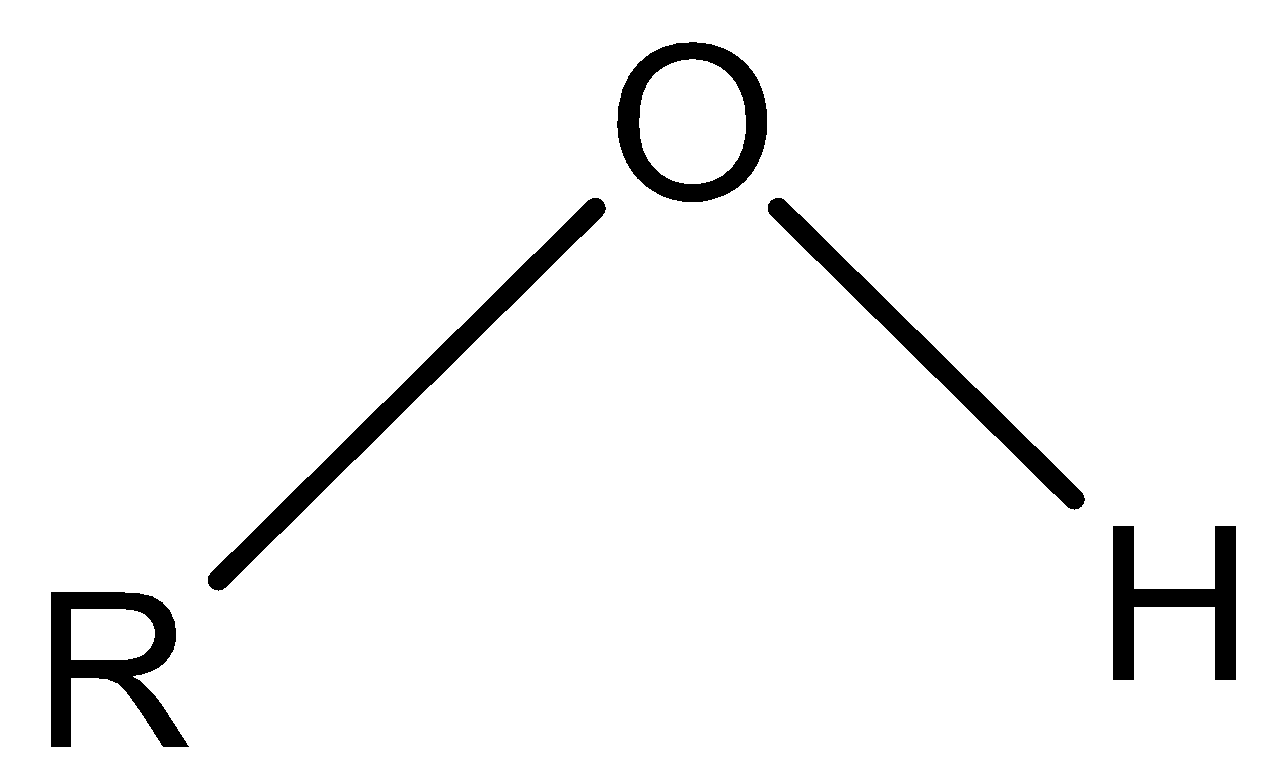
According to the rules, the hydroxy name refers to the $(.OH)$ radical only, while the $ - OH$ is named as a hydroxy group .The formation of the hydrogen bonds helps in the easy solubility of the compounds containing this group in polar solvents.
Complete step by step answer:
Pyro phosphoric acid is the inorganic compound with the formula ${H_4}{P_2}{O_7}$ . The compound is colorless and odorless and is soluble in water.
The compound is not particularly useful, except that it has the component related to the polyphosphoric acid and pyrosulphates.
Various groups attach to the compound and provide certain properties to them. One of the groups being the hydroxyl group. This group is composed of oxygen and hydrogen and can be represented as $ - OH$
Hydroxyl groups are found at the polar heads of many neutral surfactants and form the polar part of the surfactant.
This group is also contained in many compounds of carboxylic acid, alcohol, and water.
This group usually grants the property of hydrogen bonding to the group, which in turn provides properties as higher boiling point and melting points.
The structure of pyrophosphoric acid can be represented as:

From the given figure it is clear that the number of hydroxyl groups in pyrophosphoric acid is 4
So, the correct answer is Option A .
Note: The structure of the hydroxy group is represented below

According to the rules, the hydroxy name refers to the $(.OH)$ radical only, while the $ - OH$ is named as a hydroxy group .The formation of the hydrogen bonds helps in the easy solubility of the compounds containing this group in polar solvents.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




