
What will be the n-factor for $ Ba{(Mn{O_4})_2} $ in an acidic medium? (Where it behaves as oxidant)
(A) 5
(B) 10
(C) 6
(D) 3
Answer
501.3k+ views
Hint: n- Factor is the valency factor or conversion factor of a molecule. n-factor of the substance participating in a redox reaction is equal to the number of moles of electrons lost or gained by the molecule. n – factor of substance in non- redox reaction such as an acid is the number of replaceable hydrogen ions.
Complete answer:
The equation of the given molecule in a redox system where it behaves as an oxidant can be written as the following:
$ Ba{(Mn{O_4})_2} \to B{a^{2 + }} + 2MnO_4^ - $
Barium being a more electropositive element, it does not get reduced to Ba, thus only $ MnO_4^ - $ gets reduced to $ M{n^{2 + }} $ as it always does in an acidic medium.
Thus that can be represented as:
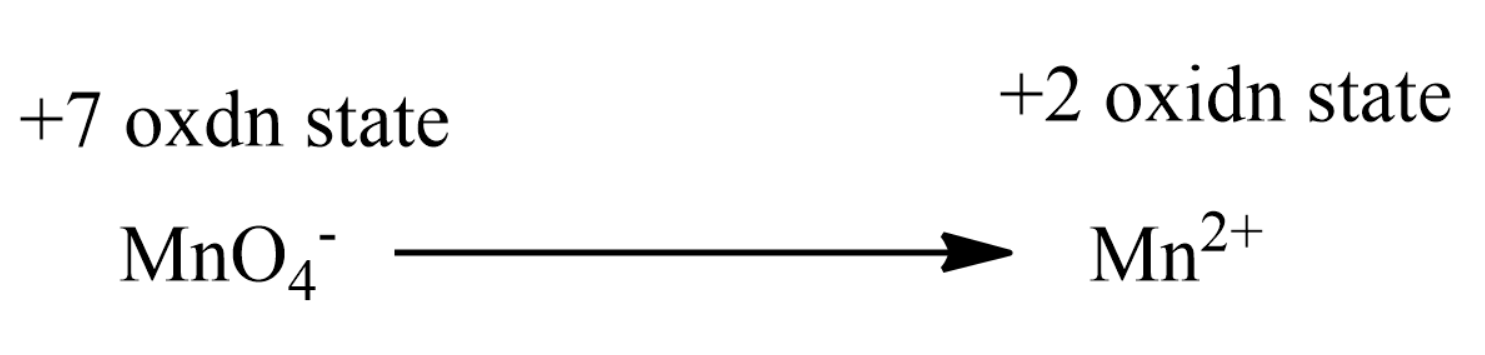
Therefore there is a net change of +5 for each molecule of $ MnO_4^ - $
Since compound has 2 molecules of $ MnO_4^ - $ in it so, the n-factor is given by,
(Change in oxidation number per atom or molecule) $ \times $ (Number of atoms per molecule)
Thus we can say that for this molecule, the n-factor is given as
$ \Rightarrow 5 \times 2 $
$ \Rightarrow 10 $
Thus we can say that the n-factor for $ Ba{(Mn{O_4})_2} $ in an acidic medium will be 10
Thus the correct option is option (2).
Note:
Be familiar with the ways in which we calculate the n-factor of different types of molecules.
For acids the n-factor will be the number of hydronium ions that can be produced.
For bases the n-factor will be the number of hydroxide ions that can be produced.
For redox reactions the n-factor will depend on the change in oxidation state of a given species.
n-factor also depends on the type and extent that the reaction undergoes and hence is not fixed for a given molecule.
Complete answer:
The equation of the given molecule in a redox system where it behaves as an oxidant can be written as the following:
$ Ba{(Mn{O_4})_2} \to B{a^{2 + }} + 2MnO_4^ - $
Barium being a more electropositive element, it does not get reduced to Ba, thus only $ MnO_4^ - $ gets reduced to $ M{n^{2 + }} $ as it always does in an acidic medium.
Thus that can be represented as:

Therefore there is a net change of +5 for each molecule of $ MnO_4^ - $
Since compound has 2 molecules of $ MnO_4^ - $ in it so, the n-factor is given by,
(Change in oxidation number per atom or molecule) $ \times $ (Number of atoms per molecule)
Thus we can say that for this molecule, the n-factor is given as
$ \Rightarrow 5 \times 2 $
$ \Rightarrow 10 $
Thus we can say that the n-factor for $ Ba{(Mn{O_4})_2} $ in an acidic medium will be 10
Thus the correct option is option (2).
Note:
Be familiar with the ways in which we calculate the n-factor of different types of molecules.
For acids the n-factor will be the number of hydronium ions that can be produced.
For bases the n-factor will be the number of hydroxide ions that can be produced.
For redox reactions the n-factor will depend on the change in oxidation state of a given species.
n-factor also depends on the type and extent that the reaction undergoes and hence is not fixed for a given molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




