
The name of the compound \[C{H_3}C{H_2}CHO\] is:
A.Propanal
B.Propanone
C.Ethanol
D.Ethanal
Answer
587.4k+ views
Hint: To know the name of a chemical compound we must know the rules of nomenclature and the structure of the compound. Having knowledge of prefix and suffix i.e. parent alkyl chain and functional group, we can easily determine the name of the compound.
Complete step by step answer:
The first basic step to name a compound is to identify the functional group present in the compound. In the given compound, the functional group present is aldehyde \[\left( { - CHO} \right)\]. The number of carbon atoms present in the compound is 3 which gives the prefix ‘prop’ to the compound.
For functional group aldehyde, the suffix to be used with alkyl chain is ‘-al’. therefore, from the suffix and prefix the name of the compound is propanal. We can also write \[C{H_3}C{H_2}CHO\] as \[{C_3}{H_6}O\]. All the carbon atoms present in the molecule are counted to identify the prefix or parent alkyl chain of a compound.
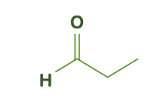
If we look at other options such as propanone, we get to know from the name that its parent alkyl chain is prop but the functional group is ketone.
If we look at ethanol and ethanal, they have the same parent alkyl chain ‘eth’ means 2 carbons but the functional groups are alcohol and aldehyde respectively.
Therefore, from all the three options given, only the first option resembles the structure provided.
Hence, the correct option is (A).
Note:
Propanal is a volatile, colourless, flammable with a suffocating fruity odour liquid substance which is readily oxidized when in contact with oxygen and thus stored under inert gases. It is produced by oxo reaction of ethylene with carbon monoxide and hydrogen.
Complete step by step answer:
The first basic step to name a compound is to identify the functional group present in the compound. In the given compound, the functional group present is aldehyde \[\left( { - CHO} \right)\]. The number of carbon atoms present in the compound is 3 which gives the prefix ‘prop’ to the compound.
For functional group aldehyde, the suffix to be used with alkyl chain is ‘-al’. therefore, from the suffix and prefix the name of the compound is propanal. We can also write \[C{H_3}C{H_2}CHO\] as \[{C_3}{H_6}O\]. All the carbon atoms present in the molecule are counted to identify the prefix or parent alkyl chain of a compound.

If we look at other options such as propanone, we get to know from the name that its parent alkyl chain is prop but the functional group is ketone.
If we look at ethanol and ethanal, they have the same parent alkyl chain ‘eth’ means 2 carbons but the functional groups are alcohol and aldehyde respectively.
Therefore, from all the three options given, only the first option resembles the structure provided.
Hence, the correct option is (A).
Note:
Propanal is a volatile, colourless, flammable with a suffocating fruity odour liquid substance which is readily oxidized when in contact with oxygen and thus stored under inert gases. It is produced by oxo reaction of ethylene with carbon monoxide and hydrogen.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




