
The most suitable method of separation of \[1:1\] mixture of ortho and para-nitrophenols is:
A. Steam distillation
B. Sublimation
C. Chromatography
D. Crystallisation
Answer
577.5k+ views
Hint: The boiling point difference between the ortho and para-nitrophenol will help us to choose the appropriate method of separation.
Complete answer
As we know that the intra and intermolecular bonding will be seen in the ortho and para-nitrophenol, this will make the molecule more stable than other and affect the boiling point. The intra and intermolecular hydrogen bonding in ortho and para-nitrophenol is shown below.
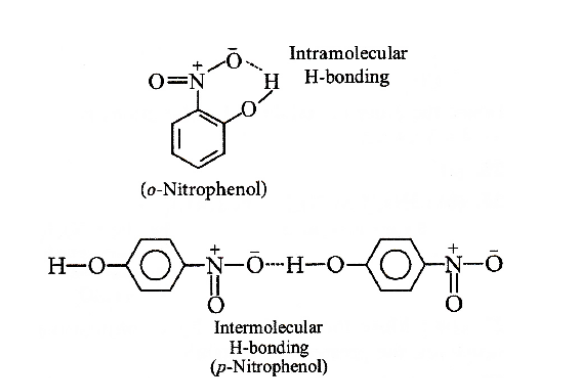
First option given here is steam distillation, in this method the boiling point difference between the molecules will play a major role in separating the mixture. The mixture with boiling point more than \[{25^ \circ }C\] is poured in the round bottom flask and it is heated at the bottom. The vapors of the liquid with lower boiling point will pass through the water condenser and will condense to liquid form that will be collected to the conical flask. The o-nitro phenol will be having intramolecular bonding i.e. the bonding is in-between the atoms of the molecules and in p-nitrophenol the intermolecular hydrogen bonding is seen which is not easy to break but intramolecular bonding is easy to break hence the boiling point of p-nitrophenol is higher than that of the o-nitrophenol. Hence during steam distillation, the o-nitrophenol will vaporize first and will be separated from the \[1:1\] mixture.
Sublimation method will be used to separate the solid particles which will not sublime easily and the particles which get converted into the gaseous phase from directly solid phase skipping the liquid phase. Separation of two solids like salt and naphthalene is done as naphthalene sublimes.
Chromatography is used to separate two liquids that move at different rates when they pass through the medium. It works on the capillary action. Hence these cannot be used to separate \[1:1\] mixture of ortho and para-nitrophenol.
Crystallization is used to purify the solid having impurities which are not soluble in the solvent in which pure substance is soluble. The impurities will remain insoluble and can be removed after dissolving the substance in the solvent to make a saturated solution and then filtering it so we get precipitate in the filter paper and the remaining solution is kept till the crystal appears after evaporation of the solvent. Hence, these cannot be used to separate \[1:1\] mixture of ortho and para-nitrophenol.
Hence, the correct answer is (A) Steam distillation.
Note:
If the question is changed to “the most suitable method of separation of \[1:2\] or \[n:n\] mixture of ortho and para-nitrophenol is”, the answer will be the same i.e. steam distillation. This means the ratio given in the question is not an important information.
Complete answer
As we know that the intra and intermolecular bonding will be seen in the ortho and para-nitrophenol, this will make the molecule more stable than other and affect the boiling point. The intra and intermolecular hydrogen bonding in ortho and para-nitrophenol is shown below.

First option given here is steam distillation, in this method the boiling point difference between the molecules will play a major role in separating the mixture. The mixture with boiling point more than \[{25^ \circ }C\] is poured in the round bottom flask and it is heated at the bottom. The vapors of the liquid with lower boiling point will pass through the water condenser and will condense to liquid form that will be collected to the conical flask. The o-nitro phenol will be having intramolecular bonding i.e. the bonding is in-between the atoms of the molecules and in p-nitrophenol the intermolecular hydrogen bonding is seen which is not easy to break but intramolecular bonding is easy to break hence the boiling point of p-nitrophenol is higher than that of the o-nitrophenol. Hence during steam distillation, the o-nitrophenol will vaporize first and will be separated from the \[1:1\] mixture.
Sublimation method will be used to separate the solid particles which will not sublime easily and the particles which get converted into the gaseous phase from directly solid phase skipping the liquid phase. Separation of two solids like salt and naphthalene is done as naphthalene sublimes.
Chromatography is used to separate two liquids that move at different rates when they pass through the medium. It works on the capillary action. Hence these cannot be used to separate \[1:1\] mixture of ortho and para-nitrophenol.
Crystallization is used to purify the solid having impurities which are not soluble in the solvent in which pure substance is soluble. The impurities will remain insoluble and can be removed after dissolving the substance in the solvent to make a saturated solution and then filtering it so we get precipitate in the filter paper and the remaining solution is kept till the crystal appears after evaporation of the solvent. Hence, these cannot be used to separate \[1:1\] mixture of ortho and para-nitrophenol.
Hence, the correct answer is (A) Steam distillation.
Note:
If the question is changed to “the most suitable method of separation of \[1:2\] or \[n:n\] mixture of ortho and para-nitrophenol is”, the answer will be the same i.e. steam distillation. This means the ratio given in the question is not an important information.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




