
The most stable conformer of Cis-1,4-cyclohexan-1,4-diol is:
A.Diaxial boat form
B.Diequatorial boat form
C.Diaxial chair form
D.Diequatorial chair form
Answer
591.3k+ views
Hint: Draw all the forms one by one and compare their stability. Also check the possibility of 1,3 diaxial repulsion and steric hindrance while assigning the stability priority.
Complete step by step answer:
We will draw the structure for every form and then we will decide which is stable or which is not. Let’s start with option A-Diaxial boat form
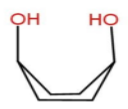
Here we can see there will be a strong intramolecular hydrogen bonding. In cyclohexane boat form 1, 4 interaction is known as Flagpole interaction which makes it unstable. But in case of Cis-1,4-cyclohexan-1,4-diol ,\[ - OH\] groups are present instead of hydrogen then there will be a strong hydrogen bonding and no flagpole interaction. Hence Diaxial boat form is stable.
Let’s move to option B – Diequatorial boat form.
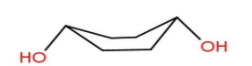
Here we can see that \[ - OH\] groups are at equilibrium but hydrogens present at axial position will show flagpole interaction. Due to this interaction Diequatorial boat form is unstable.
Now Option C- Diaxial chair form.
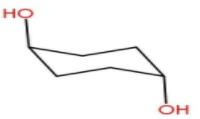
In Diaxial chair form there is a high possibility of 1, 3-Diaxial repulsion . Due to this interaction there will
be a strong steric hindrance in this form. Hence this is also unstable.
Let’s move to the last option D- Diequatorial form.
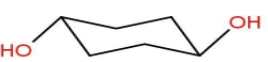
Though \[ - OH\] groups being bulky are placed at equatorial positions. Still there is a 1,3-Diaxial repulsion present in this form hence, making it unstable.
So, we have seen from all the four forms Diaxial boat form is the most stable form.
Hence the correct answer is option A.
Note:
Not every time equatorial forms will be the most stable one. There can be exceptions where axial and boat forms can be more stable than equatorial and chair forms. Take the example of 3-aminocyclohexanol. You might think that equatorial form must be a stable one because $ - N{H_2}$ is bulky than hydrogens. So if we keep the bulkier group at equatorial position it will provide stability to the compound. But that’s not the case. Here, also in axial form strong intramolecular hydrogen bonding takes place making the axial form more stable than equatorial form
Complete step by step answer:
We will draw the structure for every form and then we will decide which is stable or which is not. Let’s start with option A-Diaxial boat form

Here we can see there will be a strong intramolecular hydrogen bonding. In cyclohexane boat form 1, 4 interaction is known as Flagpole interaction which makes it unstable. But in case of Cis-1,4-cyclohexan-1,4-diol ,\[ - OH\] groups are present instead of hydrogen then there will be a strong hydrogen bonding and no flagpole interaction. Hence Diaxial boat form is stable.
Let’s move to option B – Diequatorial boat form.

Here we can see that \[ - OH\] groups are at equilibrium but hydrogens present at axial position will show flagpole interaction. Due to this interaction Diequatorial boat form is unstable.
Now Option C- Diaxial chair form.

In Diaxial chair form there is a high possibility of 1, 3-Diaxial repulsion . Due to this interaction there will
be a strong steric hindrance in this form. Hence this is also unstable.
Let’s move to the last option D- Diequatorial form.

Though \[ - OH\] groups being bulky are placed at equatorial positions. Still there is a 1,3-Diaxial repulsion present in this form hence, making it unstable.
So, we have seen from all the four forms Diaxial boat form is the most stable form.
Hence the correct answer is option A.
Note:
Not every time equatorial forms will be the most stable one. There can be exceptions where axial and boat forms can be more stable than equatorial and chair forms. Take the example of 3-aminocyclohexanol. You might think that equatorial form must be a stable one because $ - N{H_2}$ is bulky than hydrogens. So if we keep the bulkier group at equatorial position it will provide stability to the compound. But that’s not the case. Here, also in axial form strong intramolecular hydrogen bonding takes place making the axial form more stable than equatorial form
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction

State the laws of reflection of light




