
The most stable carbonium ion is:
A. Methyl carbonium ion
B. Primary carbonium ion
C. Secondary carbonium ion
D. Tertiary carbonium ion
Answer
559.5k+ views
Hint: We have to remember that a carbocation or a carbonium ion is called the cationic species in which the positive charge is borne (just like born) by carbon.
We need to know that the ${}^ + C{H_3}$is the simplest carbocation and other carbocations are named as carbocation’s derivatives. The stability of carbonium ions are explained by $ + I$ effect (electron-releasing inductive effect) and hyperconjugation effect.
Complete step by step answer:
We need to know that through the $\sigma $ bond, the displacement of electrons along the chain is called inductive effect. It is classified into two types, $ - I$ effect and $ + I$ effect.
$ + I$effect: The electron releasing group (like alkyl group), pushing the electron away from itself is called electron-releasing inductive effect or$ + I$ effect.
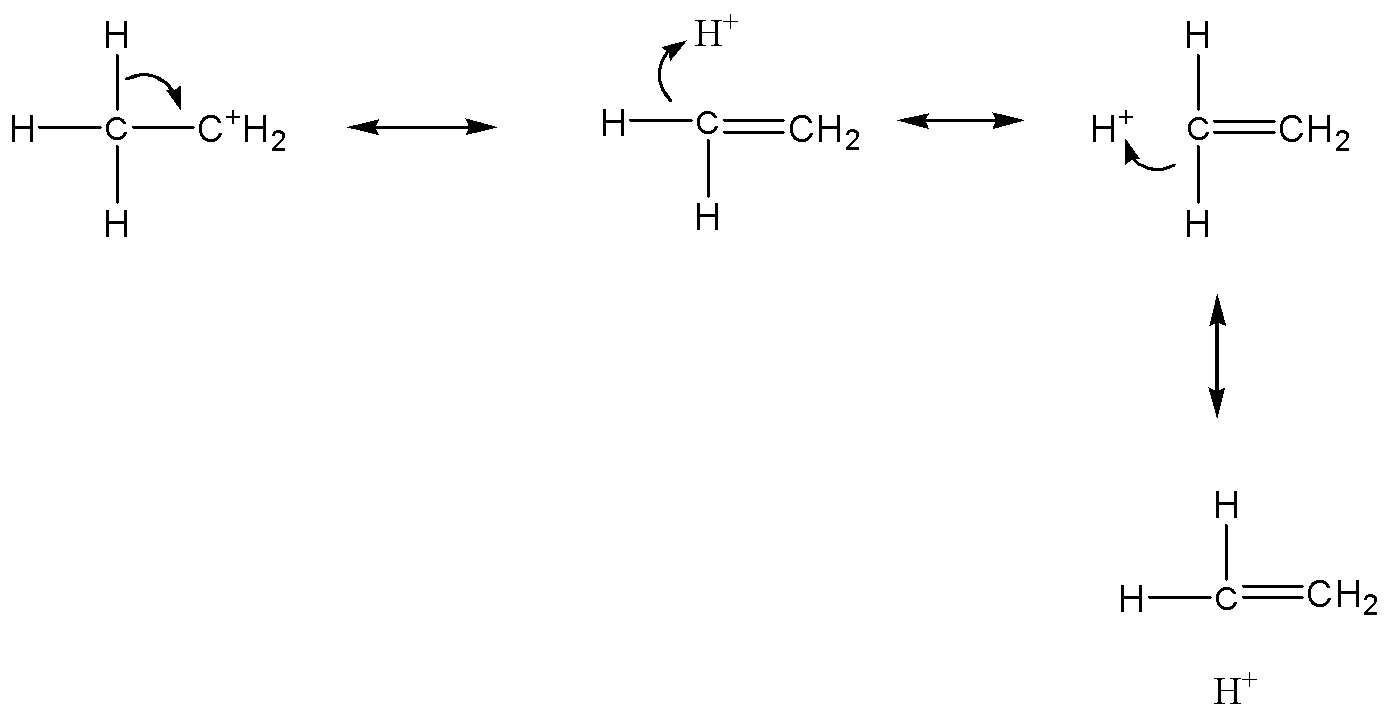
Now we can discuss the hyperconjugation effect: Between a $\pi - $orbital of carbon and a $\sigma - orbital$, the hydrogen of the methyl group, the electrons are delocalized and take overlap. This effect is also known as no bond resonance or secondary bond resonance or Baker & Nathan effect. For example:
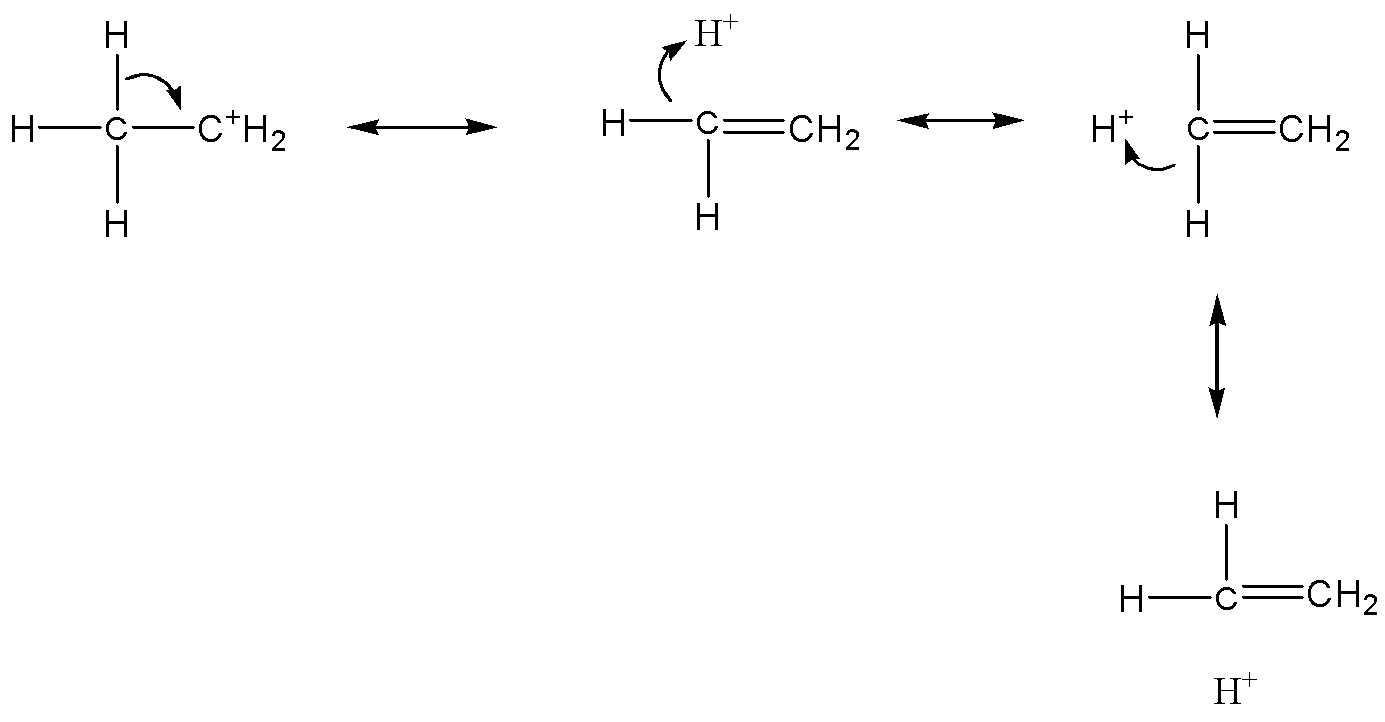
If a compound does not have hydrogen attached to adjacent carbon of $s{p^2}$ carbon, then there is no hyperconjugation. The stability of carbonium ions is explained theoretically as hyperconjugation. The electrons donated from a $\sigma - bond$ (the $C - H$ during this case) to the empty $p - $orbital, this is a more powerful concept.
If the number of alkyl groups attached to the positively charged carbon is more, then the number of hyperconjugation resonance structures is more, so the stability of carbocation also more. Among the given options, the increasing stability of carbocation follows,
Tertiary > secondary > primary > methyl
Tertiary carbocation has more resonance forms than secondary carbocation. The secondary carbocation has more resonance structures than primary carbocation.
Applying these effects to the given options, among them tertiary carbonium ion have more resonance structures, the resonance structures of the tertiary carbonium ion are follows,
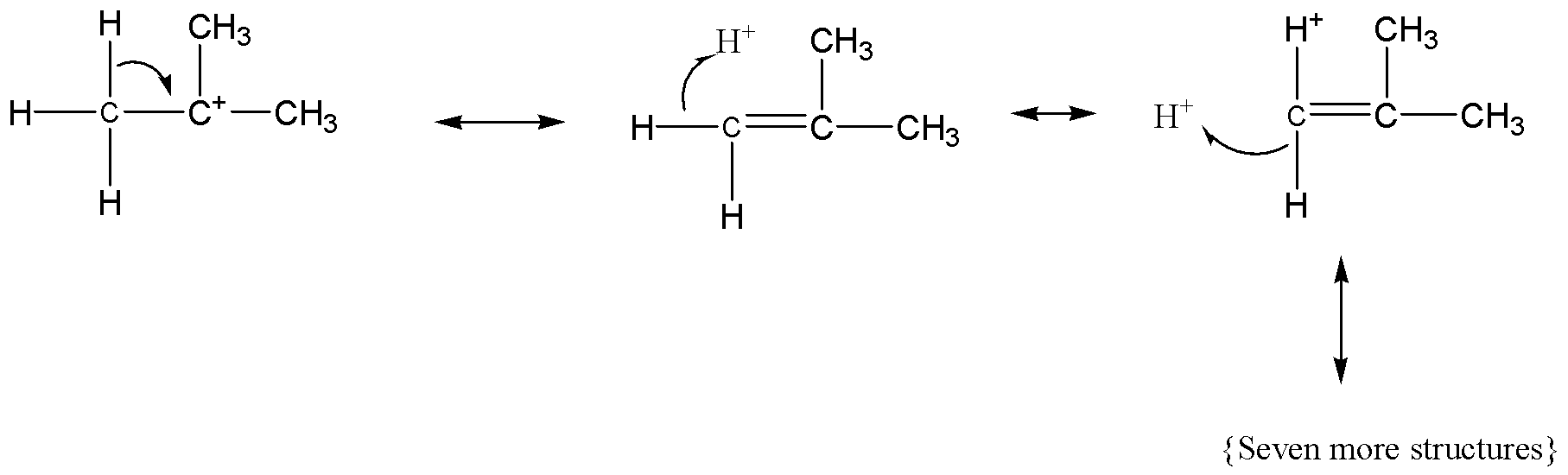
For secondary carbocation, the hyper conjugative forms are as follows,
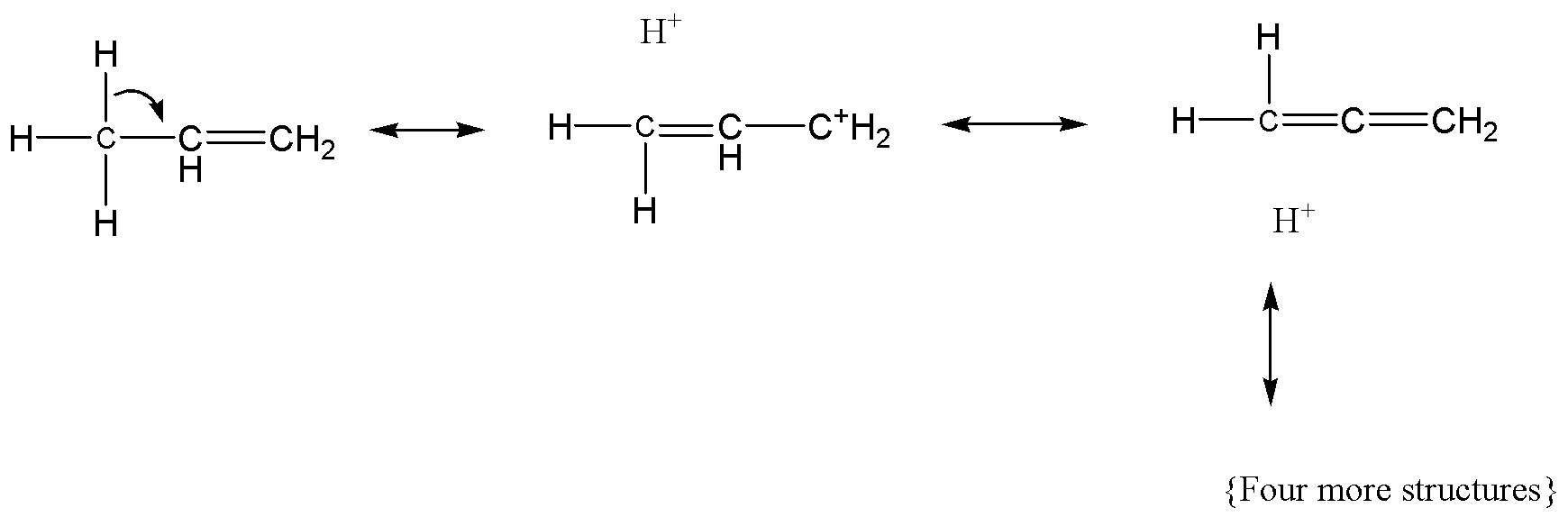
The hyper conjugative forms of primary carbocation as follows,
Methyl carbonium ion, $C{}^ + {H_3}$, does not have any hyperconjugation structures.
So, the correct answer is Option D.
Note: We need to know that the ${H^ + }$ ion in the hyper conjugative form doesn't go away, it always places in nearer to the parent carbon atom, but is not involved in bond formation. So called no bond resonance. Inductive effect is less strong than hyper conjugative effect. Hyper conjugative effect is first noted by Baker and Nathan, so called Baker & Nathan effect. The carbocations can be detected by NMR spectroscopy.
We need to know that the ${}^ + C{H_3}$is the simplest carbocation and other carbocations are named as carbocation’s derivatives. The stability of carbonium ions are explained by $ + I$ effect (electron-releasing inductive effect) and hyperconjugation effect.
Complete step by step answer:
We need to know that through the $\sigma $ bond, the displacement of electrons along the chain is called inductive effect. It is classified into two types, $ - I$ effect and $ + I$ effect.
$ + I$effect: The electron releasing group (like alkyl group), pushing the electron away from itself is called electron-releasing inductive effect or$ + I$ effect.
Now we can discuss the hyperconjugation effect: Between a $\pi - $orbital of carbon and a $\sigma - orbital$, the hydrogen of the methyl group, the electrons are delocalized and take overlap. This effect is also known as no bond resonance or secondary bond resonance or Baker & Nathan effect. For example:

If a compound does not have hydrogen attached to adjacent carbon of $s{p^2}$ carbon, then there is no hyperconjugation. The stability of carbonium ions is explained theoretically as hyperconjugation. The electrons donated from a $\sigma - bond$ (the $C - H$ during this case) to the empty $p - $orbital, this is a more powerful concept.
If the number of alkyl groups attached to the positively charged carbon is more, then the number of hyperconjugation resonance structures is more, so the stability of carbocation also more. Among the given options, the increasing stability of carbocation follows,
Tertiary > secondary > primary > methyl
Tertiary carbocation has more resonance forms than secondary carbocation. The secondary carbocation has more resonance structures than primary carbocation.
Applying these effects to the given options, among them tertiary carbonium ion have more resonance structures, the resonance structures of the tertiary carbonium ion are follows,

For secondary carbocation, the hyper conjugative forms are as follows,

The hyper conjugative forms of primary carbocation as follows,

Methyl carbonium ion, $C{}^ + {H_3}$, does not have any hyperconjugation structures.
So, the correct answer is Option D.
Note: We need to know that the ${H^ + }$ ion in the hyper conjugative form doesn't go away, it always places in nearer to the parent carbon atom, but is not involved in bond formation. So called no bond resonance. Inductive effect is less strong than hyper conjugative effect. Hyper conjugative effect is first noted by Baker and Nathan, so called Baker & Nathan effect. The carbocations can be detected by NMR spectroscopy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




