
The most common fullerene is\[{C_{60}}\]. 60 carbon atoms are arranged in _________ pentagons and __________ hexagons.
A.12 , 20
B.16 , 26
C.12 , 40
D.16 , 40
Answer
576k+ views
Hint: \[{C_{60}}\] is the third allotropic form of carbon. It has a cage-like fused ring structure that looks like a soccer ball.
Complete step by step answer:
Fullerene-It is an allotrope of carbon in which 60 carbons are attached in a hollow sphere (bulky balls) or in a tube like structure. It is also known as Buckminsterfullerene. It contains both single and double bonds. The general formula for Fullerene is \[{C_n}\] where \[n\] is the number of carbon atoms and it should be greater than or equal to 30.
Preparation-
\[
graphite\xrightarrow[{He}]{{electric\,arc}}vapourisation \\
\xrightarrow{{condensation}}fullerene\,set \\
\xrightarrow{{chromatography}}{C_{60}} + {C_{70}} \\
\]
Fullerene is the purest form of carbon because they don’t have any surface bonds just like graphite and diamond.
Structure:
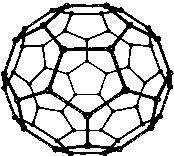
\[{C_{60}}\] is the most stable form of carbon and looks like a soccer ball. It consists of 20 six membered rings and 12 five membered rings. In fullerene five membered rings are connected to six membered rings only but six membered are fused to both six membered and five membered rings.
So the correct answer is option A-60 carbon atoms are arranged in 12 pentagons and 60 hexagons.
Note:
As all the bonds are in between carbon atoms, the strain caused by the hindrance leads to the distortion in coplanarity of the molecule .This strain is distributed equally to all the carbon atoms.Fullerenes have many pharmaceutical applications. It can also be used as a radical scavenger and antioxidant.This can be used as a catalyst and in lubricants.
Complete step by step answer:
Fullerene-It is an allotrope of carbon in which 60 carbons are attached in a hollow sphere (bulky balls) or in a tube like structure. It is also known as Buckminsterfullerene. It contains both single and double bonds. The general formula for Fullerene is \[{C_n}\] where \[n\] is the number of carbon atoms and it should be greater than or equal to 30.
Preparation-
\[
graphite\xrightarrow[{He}]{{electric\,arc}}vapourisation \\
\xrightarrow{{condensation}}fullerene\,set \\
\xrightarrow{{chromatography}}{C_{60}} + {C_{70}} \\
\]
Fullerene is the purest form of carbon because they don’t have any surface bonds just like graphite and diamond.
Structure:

\[{C_{60}}\] is the most stable form of carbon and looks like a soccer ball. It consists of 20 six membered rings and 12 five membered rings. In fullerene five membered rings are connected to six membered rings only but six membered are fused to both six membered and five membered rings.
So the correct answer is option A-60 carbon atoms are arranged in 12 pentagons and 60 hexagons.
Note:
As all the bonds are in between carbon atoms, the strain caused by the hindrance leads to the distortion in coplanarity of the molecule .This strain is distributed equally to all the carbon atoms.Fullerenes have many pharmaceutical applications. It can also be used as a radical scavenger and antioxidant.This can be used as a catalyst and in lubricants.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




