
The molecule that deviates from octet rule is:
A. $NaCl$
B. $BeCl{}_{2}$
C. $BH{}_{4}$
D. $NH{}_{3}$
Answer
562.8k+ views
Hint: The chemical rule of thumb that reflects the observation of the main group of elements tends to bond in a way that each atom has eight electrons in their valence shell is known as the octet rule. Chemical bonding is a good example of the octet rule.
Complete step by step answer:
So, they form the octet. First we will discuss about the octet rule,
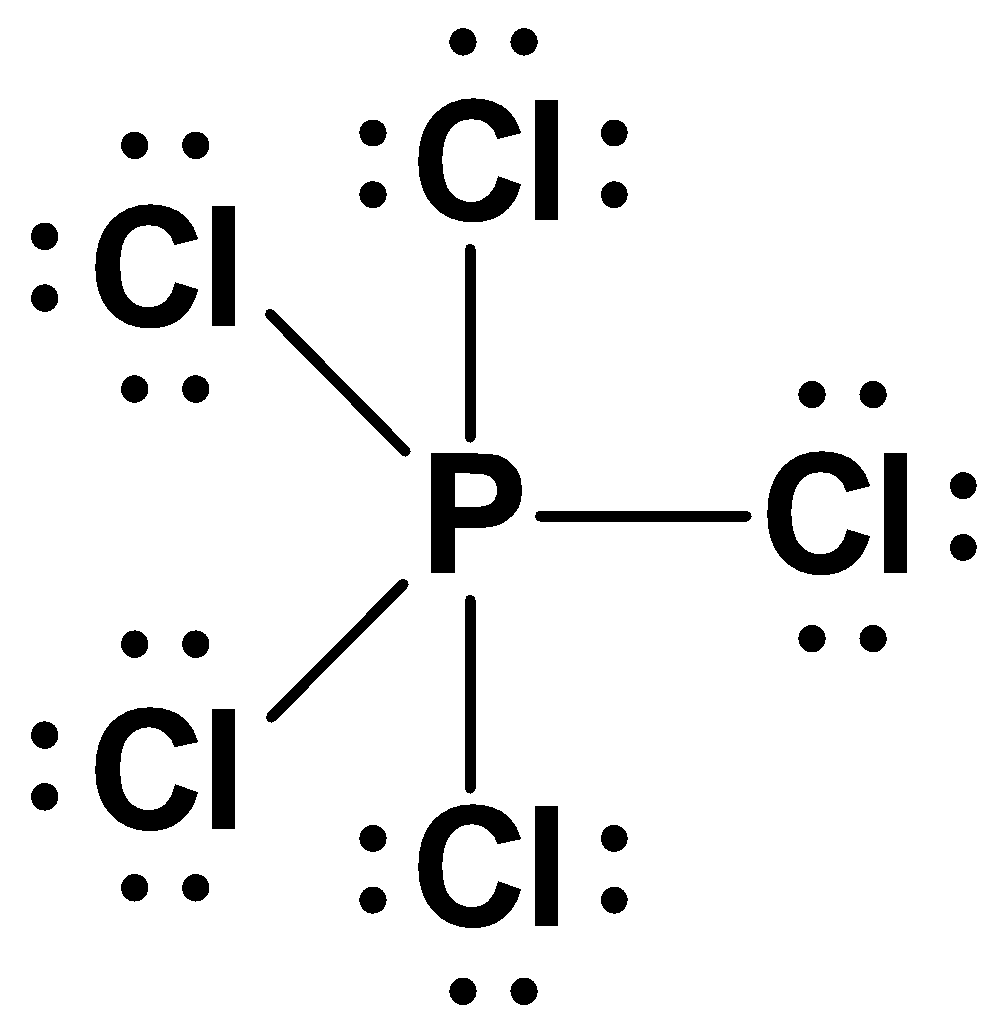
Octet rule is defined as when the atoms combine to form molecules they generally each lose, gain or share valence electrons until they attain or share eight electrons. The octet rule is also called the Lewis rule of eight. For example: - Phosphorus pentachloride ($PCl{}_{5}$) is an example of octet rule by having more than 8 electrons in the valence shell. The structure is as follows:
Abegg’s rule was formulated by Richard Abegg in 1904, which states that the difference between the maximum positive and negative valences of an element is frequently eight.
Now according to the question , $BeCl{}_{2}$ is deviated the octet rule, the structure can be represented as follows:
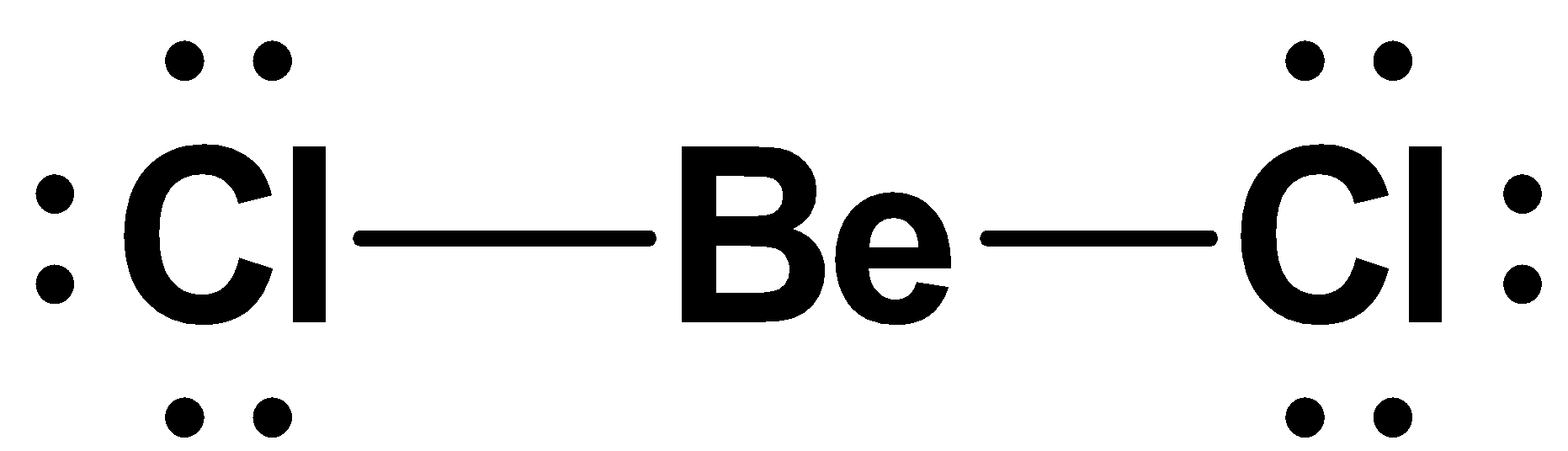
Because in $BeCl{}_{2}$ the beryllium loss its two electrons to chlorine so that chlorine has eight electrons in the valence shell and $NaCl$ is the ionic compound and is formed by the complete transfers of electrons between the electro positive and negative elements. The $Na$ or $Cl$ are surrounded by eight electrons. The structure can be represented as follows:
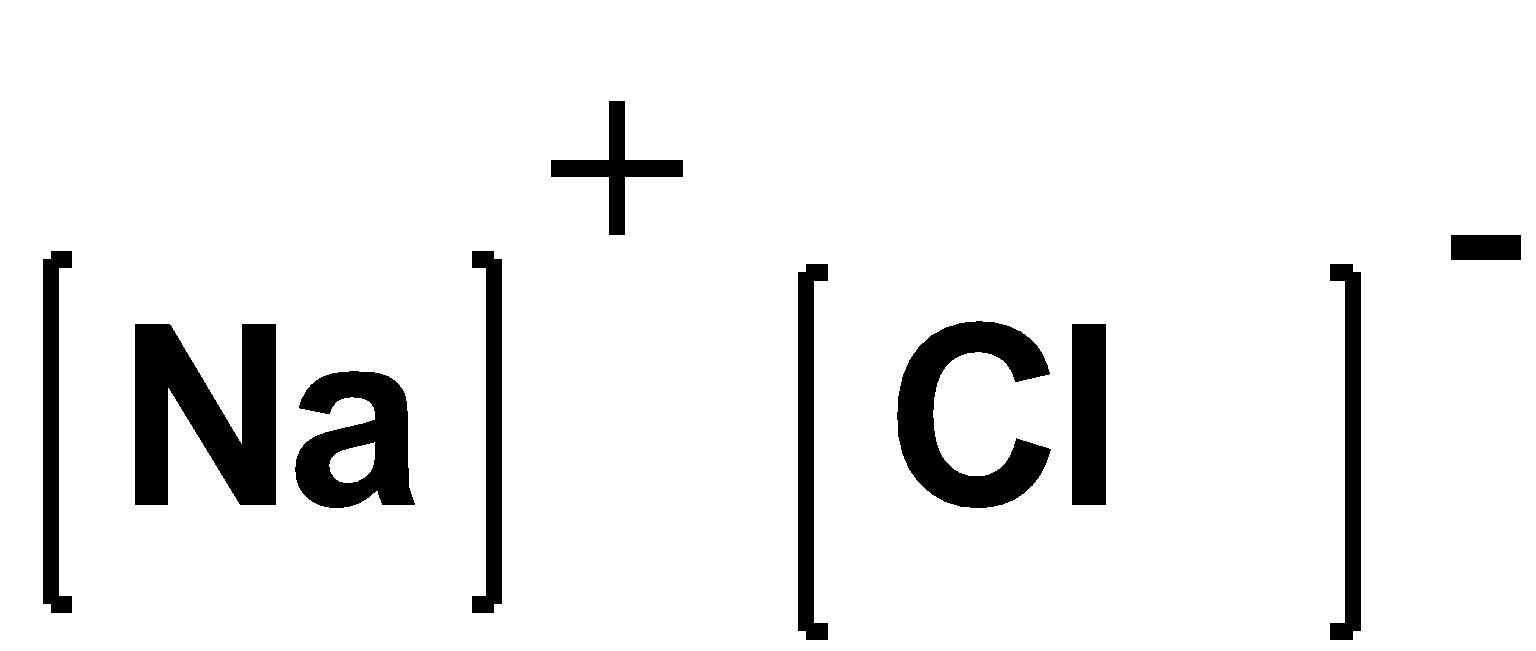
Therefore, option B is correct.
Note: The covalent bonding is important in the octet rule because sharing electrons gives both atoms a full valence shell. This is the most stable electron arrangement. Each atom can count the shared electrons as part of its own valence shell and is known as the covalent bonding.
Complete step by step answer:
So, they form the octet. First we will discuss about the octet rule,
Octet rule is defined as when the atoms combine to form molecules they generally each lose, gain or share valence electrons until they attain or share eight electrons. The octet rule is also called the Lewis rule of eight. For example: - Phosphorus pentachloride ($PCl{}_{5}$) is an example of octet rule by having more than 8 electrons in the valence shell. The structure is as follows:

Abegg’s rule was formulated by Richard Abegg in 1904, which states that the difference between the maximum positive and negative valences of an element is frequently eight.
Now according to the question , $BeCl{}_{2}$ is deviated the octet rule, the structure can be represented as follows:
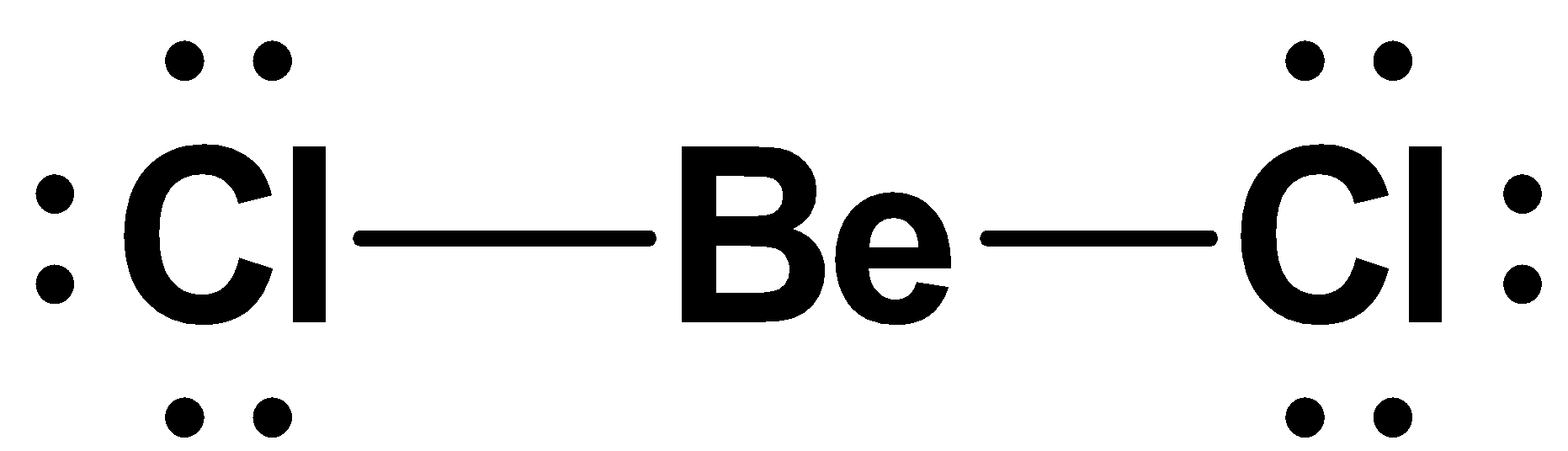
Because in $BeCl{}_{2}$ the beryllium loss its two electrons to chlorine so that chlorine has eight electrons in the valence shell and $NaCl$ is the ionic compound and is formed by the complete transfers of electrons between the electro positive and negative elements. The $Na$ or $Cl$ are surrounded by eight electrons. The structure can be represented as follows:
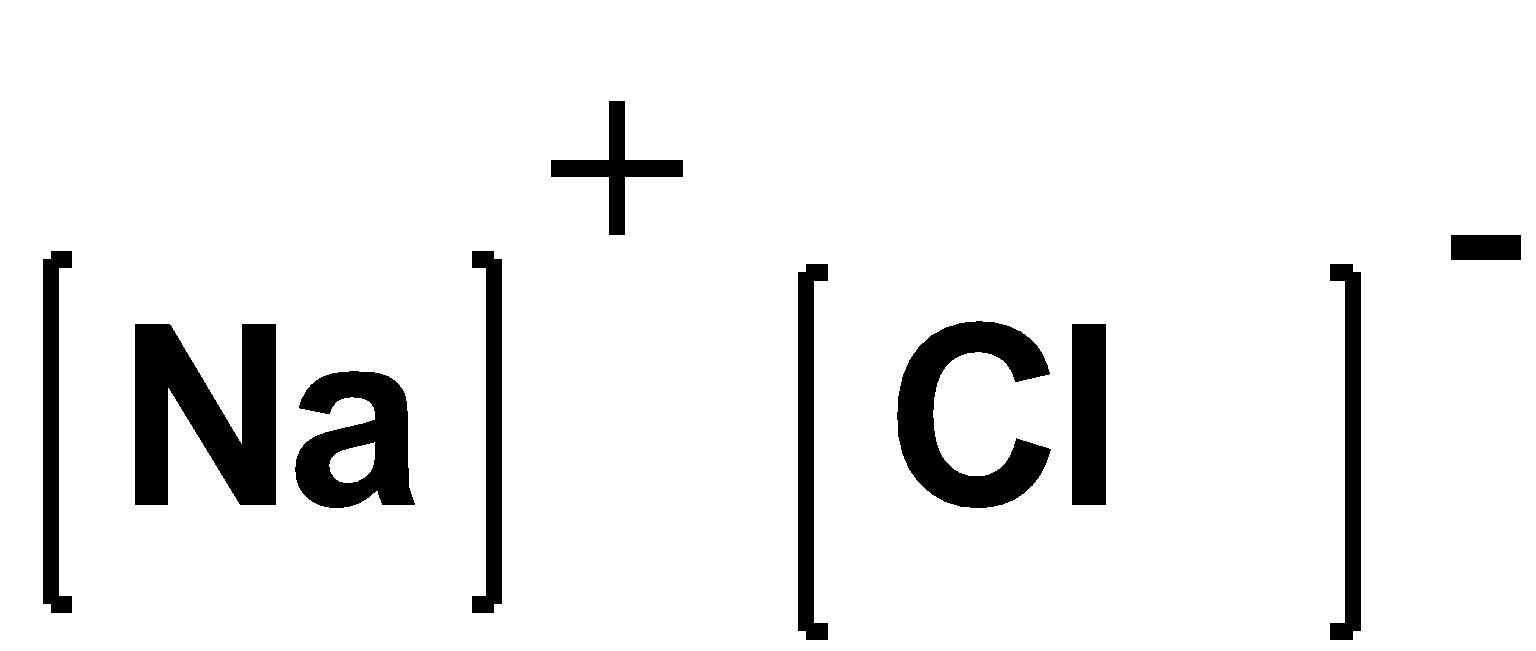
Therefore, option B is correct.
Note: The covalent bonding is important in the octet rule because sharing electrons gives both atoms a full valence shell. This is the most stable electron arrangement. Each atom can count the shared electrons as part of its own valence shell and is known as the covalent bonding.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




