
The molecule of magnesium chloride is linear, whereas that of stannous chloride is angular.
If true enter 1, else enter 0.
Answer
583.8k+ views
Hint: First check the number of valence electrons in Mg and Sn atoms of both compounds and their bonding with the 2 Cl atoms. Also check for the presence of any lone pairs and try to deduce the structure. Calculate the hybridisation using the formula given below and see the structure related to it.
$H = 1/2\left[ {V + M - C + A} \right]$
Complete step by step answer:
-First of all we will start by calculating the hybridisation of magnesium chloride ($MgC{l_2}$) and checking its shape. For finding out the hybridisation we will use the following formula:
$H = 1/2\left[ {V + M - C + A} \right]$ (1)
Where, V=number of valence electrons;
M=monovalent atoms;
C=positive charge;
A=negative charge.
Hybridisation of $MgC{l_2}$ will be: $H = \dfrac{1}{2}\left[ {2 + 2 - 0 + 0} \right]$
= $\dfrac{4}{2}$ = 2
Since the hybridisation number for it is 2, its hybridisation will be sp and its shape will be linear.
This can also be explained as: Mg has 2 electron clouds formed from its bonding with the 2 Cl atoms. The chlorine atom being highly electronegative attracts an electron from the magnesium atom and forms a bond. Also the number of valence electrons in Mg is only 2 and both are involved in bonding with 2 Cl atoms, so there are no lone pairs. Its structure is one-dimensional and the atoms are connected in straight lines. Also the bond angle here is 180$^ \circ $ . Its structure will be:

So, we can finally say that the structure of magnesium chloride ($MgC{l_2}$) is linear.
-We will now talk about the structure of stannous chloride ($SnC{l_2}$).
First let us calculate its hybridisation using equation (1).
$H = \dfrac{1}{2}\left[ {4 + 2 - 0 + 0} \right]$
= 3
The hybridisation number is 3 and so hybridisation will be $s{p^2}$. Out of the 4 valence electrons in Sn, 2 are bonded with the 2 Cl atoms and there is a lone pair of electrons left. So, it forms a tetrahedral structure.
This can also be explained as: $SnC{l_2}$ is an angular covalent molecule due to the repulsion between the lone pair of electrons on Sn atom and the two bonds with the 2 chlorine atoms.
Its structure will be:
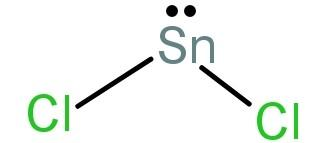
-As we have proved above the given statement: “The molecule of magnesium chloride is linear, whereas that of stannous chloride is angular” is true.
Note: Hybridisation does not only depend on the number of bonds formed in the given compound. The presence or absence of any lone pairs also plays a major role in it because the presence of lone pair causes lone pair-bond pair repulsion leading to the angularity of the structures.
$H = 1/2\left[ {V + M - C + A} \right]$
Complete step by step answer:
-First of all we will start by calculating the hybridisation of magnesium chloride ($MgC{l_2}$) and checking its shape. For finding out the hybridisation we will use the following formula:
$H = 1/2\left[ {V + M - C + A} \right]$ (1)
Where, V=number of valence electrons;
M=monovalent atoms;
C=positive charge;
A=negative charge.
Hybridisation of $MgC{l_2}$ will be: $H = \dfrac{1}{2}\left[ {2 + 2 - 0 + 0} \right]$
= $\dfrac{4}{2}$ = 2
Since the hybridisation number for it is 2, its hybridisation will be sp and its shape will be linear.
This can also be explained as: Mg has 2 electron clouds formed from its bonding with the 2 Cl atoms. The chlorine atom being highly electronegative attracts an electron from the magnesium atom and forms a bond. Also the number of valence electrons in Mg is only 2 and both are involved in bonding with 2 Cl atoms, so there are no lone pairs. Its structure is one-dimensional and the atoms are connected in straight lines. Also the bond angle here is 180$^ \circ $ . Its structure will be:

So, we can finally say that the structure of magnesium chloride ($MgC{l_2}$) is linear.
-We will now talk about the structure of stannous chloride ($SnC{l_2}$).
First let us calculate its hybridisation using equation (1).
$H = \dfrac{1}{2}\left[ {4 + 2 - 0 + 0} \right]$
= 3
The hybridisation number is 3 and so hybridisation will be $s{p^2}$. Out of the 4 valence electrons in Sn, 2 are bonded with the 2 Cl atoms and there is a lone pair of electrons left. So, it forms a tetrahedral structure.
This can also be explained as: $SnC{l_2}$ is an angular covalent molecule due to the repulsion between the lone pair of electrons on Sn atom and the two bonds with the 2 chlorine atoms.
Its structure will be:

-As we have proved above the given statement: “The molecule of magnesium chloride is linear, whereas that of stannous chloride is angular” is true.
Note: Hybridisation does not only depend on the number of bonds formed in the given compound. The presence or absence of any lone pairs also plays a major role in it because the presence of lone pair causes lone pair-bond pair repulsion leading to the angularity of the structures.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




