
The molecular shape of $S{{F}_{4}}$, $C{{F}_{4}}$ and $Xe{{F}_{4}}$ are:
(A) the same, with 2, 0 and 1 lone pair of electrons respectively.
(B) the same, with 1, 1 and 1 lone pair of electrons respectively.
(C) different with 0, 1 and 2 lone pairs of electrons respectively.
(D) different with 1, 0 and 2 lone pairs of electrons respectively.
Answer
569.4k+ views
Hint: The number of lone pairs and the number of bond pairs determines the shape of the given molecule. We should find the number of lone pairs on the central atom and bond pairs to determine its shape.
Complete Solution :
With help of VSEPR theory we can find the shape.
Postulates of VSEPR theory are:
-The spatial arrangement of the molecules depends upon the number of valence shell electron pairs around the central atom.
-The electron pair surrounding the central atom repels one another and moves apart from each other so that there is no further repulsion between them. As a result, the molecules have minimum energy and maximum stability.
-There are two types of electron pairs, one bond pairs and other lone pairs. The bond pairs of electrons are those shared between two atoms while the lone pair are the valence electron pairs that are not involved in bonding.
-Order of the repulsion between the electron pairs are: $lp-lp > lp-bp > bp-bp$ where, lp is lone pair and bp is bond pair.
$S{{F}_{4}}$
The valence electron in sulphur is 6.
From the chemical formula, there are 4 fluorine atoms that are chemically bonded with sulphur. Therefore, the extra 2 electrons are lone pair electrons. Thus, $S{{F}_{4}}$ has 1 lone pair.
It contains 1 lone pair and 4 bond pairs.
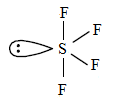
The shape is bipyramidal .
$C{{F}_{4}}$
The valence electrons is 4.
There are 4 fluorine atoms that make bonds with the valence electrons of carbon. Thus, has no lone pairs.
It contains 0 lone pairs and 4 bond pairs.
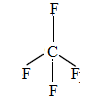
The shape is tetrahedral.
$Xe{{F}_{4}}$
The valence electrons of Xe is 8.
There are 4 fluorines that will be chemically bonded to Xe. Then there are 4 remaining electrons. This means there are two lone pairs of electrons.
It has 2 lone pairs and 4 bond pairs.
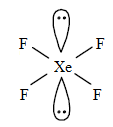
The shape is square planar.
So, the correct answer is “Option D”.
Note: Lewis dot structure is entirely based on octet rule and it failed to explain certain molecules such as ${{H}_{2}}$, $C{{l}_{2}}$, $I{{F}_{3}}$, $PC{{l}_{5}}$ ,$S{{F}_{6}}$, $B{{F}_{3}}$, $BC{{l}_{3}}$, $Xe{{F}_{4}}$ etc. VSEPR theory based on the number electron pair around the central atom, repulsion between the electron bonds around the central atom and succeeded to explain all the molecules above mentioned that Lewis theory failed to explain.
Complete Solution :
With help of VSEPR theory we can find the shape.
Postulates of VSEPR theory are:
-The spatial arrangement of the molecules depends upon the number of valence shell electron pairs around the central atom.
-The electron pair surrounding the central atom repels one another and moves apart from each other so that there is no further repulsion between them. As a result, the molecules have minimum energy and maximum stability.
-There are two types of electron pairs, one bond pairs and other lone pairs. The bond pairs of electrons are those shared between two atoms while the lone pair are the valence electron pairs that are not involved in bonding.
-Order of the repulsion between the electron pairs are: $lp-lp > lp-bp > bp-bp$ where, lp is lone pair and bp is bond pair.
$S{{F}_{4}}$
The valence electron in sulphur is 6.
From the chemical formula, there are 4 fluorine atoms that are chemically bonded with sulphur. Therefore, the extra 2 electrons are lone pair electrons. Thus, $S{{F}_{4}}$ has 1 lone pair.
It contains 1 lone pair and 4 bond pairs.

The shape is bipyramidal .
$C{{F}_{4}}$
The valence electrons is 4.
There are 4 fluorine atoms that make bonds with the valence electrons of carbon. Thus, has no lone pairs.
It contains 0 lone pairs and 4 bond pairs.

The shape is tetrahedral.
$Xe{{F}_{4}}$
The valence electrons of Xe is 8.
There are 4 fluorines that will be chemically bonded to Xe. Then there are 4 remaining electrons. This means there are two lone pairs of electrons.
It has 2 lone pairs and 4 bond pairs.

The shape is square planar.
So, the correct answer is “Option D”.
Note: Lewis dot structure is entirely based on octet rule and it failed to explain certain molecules such as ${{H}_{2}}$, $C{{l}_{2}}$, $I{{F}_{3}}$, $PC{{l}_{5}}$ ,$S{{F}_{6}}$, $B{{F}_{3}}$, $BC{{l}_{3}}$, $Xe{{F}_{4}}$ etc. VSEPR theory based on the number electron pair around the central atom, repulsion between the electron bonds around the central atom and succeeded to explain all the molecules above mentioned that Lewis theory failed to explain.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




