
The molecular formula of phosphorus is:
A.${P_1}$
B.${P_4}$
C.${P_2}$
D.${P_5}$
Answer
585.3k+ views
Hint:Phosphorus is highly reactive and is not found as an element on Earth. It can form three bonds and by this we are able to arrange it in such a way as to get the most stability.
Complete step by step answer:
Phosphorus is a chemical element with symbol P and it has atomic number 15. It lies p block in group $15$ (pnictogens) and in period 3. It falls in the category of reactive non-metal. Electronic configuration of Phosphorus is $\left[ {Ne} \right]3{s^2}3{p^3}$.
Basically, Elemental Phosphorus exists in two major forms white Phosphorus and red Phosphorus.
It is highly reactive and it is not found in an elemental form on Earth. It has a concentration of about one gram per kilogram. Phosphorus generally occurs as phosphate.
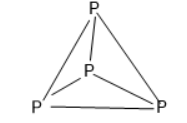
The molecular formula of Phosphorus is ${P_4}$ and exists as white Phosphorus in gaseous state and as waxy solid. It has a tetrahedral shape.
Phosphorus can form ${P_4}$(white Phosphorus) because it can form three bonds while sulfur can only form two bonds. The most stable allotrope of Phosphorus is red Phosphorus which has a cross linked polymeric chain of atoms.
Phosphorus (white) dry and under water in solution appears as a soft waxy solid and has a sharp pungent odor that is similar to garlic. It is insoluble in water and ethyl alcohol, soluble in carbon disulfide. It is denser than water and hence sinks in water.
Therefore, Option B is correct and the molecular formula of Phosphorus is $P_4$.
Note:
Phosphorus has been found in the human brain, liver and also in kidney tissues. It is also primarily detected in saliva, blood, urine. It exists in all eukaryotes ranging from yeast to humans.
It is used to manufacture smoke bombs. Artificial fertilizers and rat poisons.
Complete step by step answer:
Phosphorus is a chemical element with symbol P and it has atomic number 15. It lies p block in group $15$ (pnictogens) and in period 3. It falls in the category of reactive non-metal. Electronic configuration of Phosphorus is $\left[ {Ne} \right]3{s^2}3{p^3}$.
Basically, Elemental Phosphorus exists in two major forms white Phosphorus and red Phosphorus.
It is highly reactive and it is not found in an elemental form on Earth. It has a concentration of about one gram per kilogram. Phosphorus generally occurs as phosphate.
The molecular formula of Phosphorus is ${P_4}$ and exists as white Phosphorus in gaseous state and as waxy solid. It has a tetrahedral shape.
Phosphorus can form ${P_4}$(white Phosphorus) because it can form three bonds while sulfur can only form two bonds. The most stable allotrope of Phosphorus is red Phosphorus which has a cross linked polymeric chain of atoms.
Phosphorus (white) dry and under water in solution appears as a soft waxy solid and has a sharp pungent odor that is similar to garlic. It is insoluble in water and ethyl alcohol, soluble in carbon disulfide. It is denser than water and hence sinks in water.
Therefore, Option B is correct and the molecular formula of Phosphorus is $P_4$.

Note:
Phosphorus has been found in the human brain, liver and also in kidney tissues. It is also primarily detected in saliva, blood, urine. It exists in all eukaryotes ranging from yeast to humans.
It is used to manufacture smoke bombs. Artificial fertilizers and rat poisons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




