
The mixture of statement in respect of structure of hypo phosphorous acid is:
A. 2-OH groups, 2-H atoms are attached directly to P
B. One OH group and 2-H atoms are directly attached to P
C. One OH group and 3- H atoms are directly attached to P
D. Three OH groups are attached directly to P.
Answer
533.1k+ views
Hint: The other name of hypo phosphorous acid is phosphinic acid. Hypo phosphorous acid is a strong reducing agent. Hypo phosphorous acid has a property to get soluble in solvents like dioxane, water and alcohol.
Complete answer:
- In the question it is asked about the number of –OH groups and number of hydrogen atoms attached to hypo phosphorous acid.
- To know about the –OH groups and number of hydrogen atoms we should know the structure of the hypo phosphorous acid.
- The chemical formula of hypo phosphorous acid is ${{H}_{3}}P{{O}_{2}}$ .
- The structure of hypo phosphorous acid is as follows.
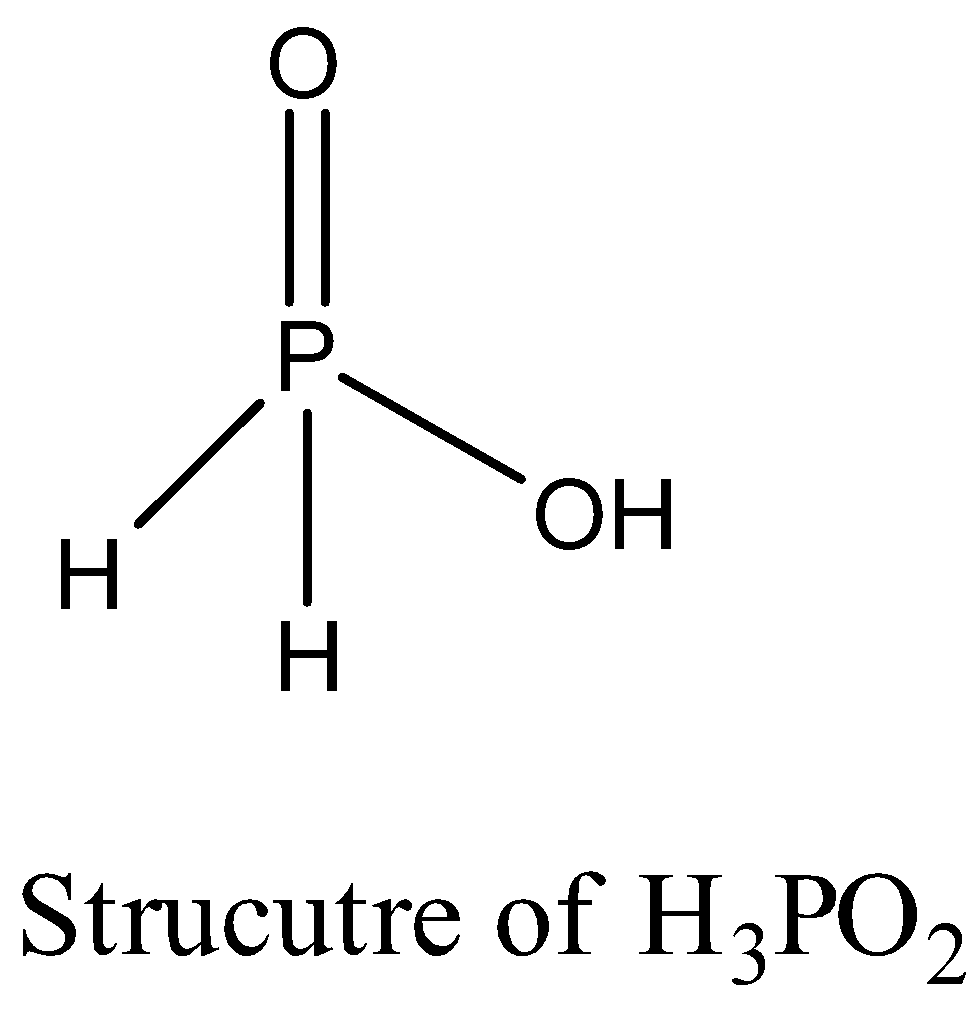
- In the above structure we can see clearly that there is one –OH group and two hydrogen atoms attached directly to the phosphorus atom.
- Therefore in the structure of hypo phosphorous acid One OH group and 2-H atoms are directly attached to P.
So, the correct option is B.
Note:
Hypo phosphorous acid is going to donate only one hydrogen atom, which is attached to oxygen. Hypophosphorous acid is going to be used in fischer esterification as an additive. In chemical reactions hypophosphorous acid is going to act as a reducing agent.
Complete answer:
- In the question it is asked about the number of –OH groups and number of hydrogen atoms attached to hypo phosphorous acid.
- To know about the –OH groups and number of hydrogen atoms we should know the structure of the hypo phosphorous acid.
- The chemical formula of hypo phosphorous acid is ${{H}_{3}}P{{O}_{2}}$ .
- The structure of hypo phosphorous acid is as follows.

- In the above structure we can see clearly that there is one –OH group and two hydrogen atoms attached directly to the phosphorus atom.
- Therefore in the structure of hypo phosphorous acid One OH group and 2-H atoms are directly attached to P.
So, the correct option is B.
Note:
Hypo phosphorous acid is going to donate only one hydrogen atom, which is attached to oxygen. Hypophosphorous acid is going to be used in fischer esterification as an additive. In chemical reactions hypophosphorous acid is going to act as a reducing agent.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




