
The mesomeric effect of the groups respectively is:
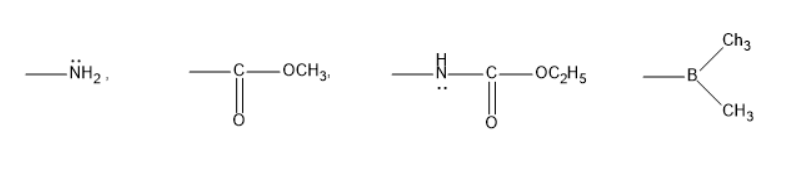
A.\[ + M, + M, - M, - M\]
B.\[ + M, - M, + M, - M\]
C.\[ - M, - M, + M, - M\]
D.\[ + M, - M, - M, + M\]
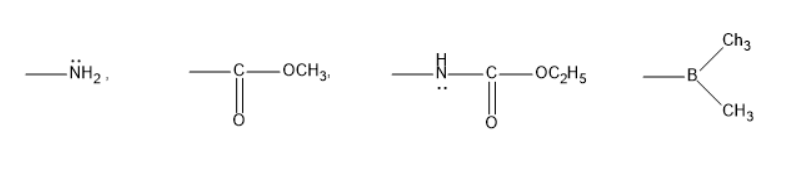
Answer
460.8k+ views
Hint: The movement of electrons is known as polarity. The polarity is produced in the molecule by the interaction of two pi-bonds or between a pi bond and lone pair of electrons present on an adjacent atom. The molecule consisting of lone pair of electrons results in the positive Mesomeric effect and the presence of electron-withdrawing groups results in the negative Mesomeric effect
Complete answer:
Chemical compounds are classified into functional groups based on the groups or atoms present in them. Some of the functional groups are amines, esters, and amides.
Amines are chemical compounds consisting of a nitrogen atom with a lone pair of electrons. These lone pairs of electrons can result in the donation and produce a positive Mesomeric effect \[ + M\] . Thus, \[ - N{H_2}\] exhibits \[ + M\] effect.
Esters are the chemical compounds consisting of the carbonyl group attached to the oxygen atom. Due to the high electronegativity of oxygen compared to carbon, it exhibits \[ - M\] effect.
Thus, \[ - COOC{H_3}\] exhibits \[ - M\] effect.
The molecule \[ - NHCOO{C_2}{H_5}\] also has a nitrogen atom with the presence of a lone pair of electrons. It exhibits positive Mesomeric effect \[ + M\].
The molecule \[ - B{(C{H_3})_2}\] has a vacant orbital resulting in the negative Mesomeric effect. Thus, it exhibits \[ - M\] effect.
Thus, Option B is the correct one.
Note:
The positive Mesomeric effect can be given by the lone pair of electrons, and the negative Mesomeric effect can be given by the compounds consisting of electronegativity difference between the atoms present in the molecule like carbonyl groups.
Complete answer:
Chemical compounds are classified into functional groups based on the groups or atoms present in them. Some of the functional groups are amines, esters, and amides.
Amines are chemical compounds consisting of a nitrogen atom with a lone pair of electrons. These lone pairs of electrons can result in the donation and produce a positive Mesomeric effect \[ + M\] . Thus, \[ - N{H_2}\] exhibits \[ + M\] effect.
Esters are the chemical compounds consisting of the carbonyl group attached to the oxygen atom. Due to the high electronegativity of oxygen compared to carbon, it exhibits \[ - M\] effect.
Thus, \[ - COOC{H_3}\] exhibits \[ - M\] effect.
The molecule \[ - NHCOO{C_2}{H_5}\] also has a nitrogen atom with the presence of a lone pair of electrons. It exhibits positive Mesomeric effect \[ + M\].
The molecule \[ - B{(C{H_3})_2}\] has a vacant orbital resulting in the negative Mesomeric effect. Thus, it exhibits \[ - M\] effect.
Thus, Option B is the correct one.
Note:
The positive Mesomeric effect can be given by the lone pair of electrons, and the negative Mesomeric effect can be given by the compounds consisting of electronegativity difference between the atoms present in the molecule like carbonyl groups.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




