
The Maxwell-Boltzmann distribution law of molecular speeds of graphically represented as:
This curve has which of the following characteristics?
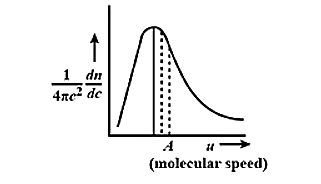
(A) It has symmetrical distribution.
(B) The point A on x-axis represents the most probable speed
(C) The area under the curve gives the total number of molecules
(D) The maximum shifts to the right as the temperature increases.

Answer
570k+ views
Hint: The motion of molecules is extremely chaotic. One individual molecule will be colliding with another molecule at an enormous rate. The rate of the collision is a billion times per second.
Complete Solution :
Let us see what a Boltzmann distribution law is.
The motion of molecules is extremely chaotic. One individual molecule will be colliding with another molecule at an enormous rate. The rate of the collision is a billion times per second. Let us introduce the term \[{\rm{nv(E)}}\] which is the number density. This is also known as the distribution function. \[dE\]is the number of molecules that are present per unit volume whose energy is between \[E\] and \[E + dE\].
Based on the statistical mechanics, we can give :
\[{\rm{nv(E) = }}{{\rm{n}}_0}{e^{\frac{{ - E}}{{{k_B}T}}}}\]
According to the Boltzmann Law, the probability of finding a molecule at a particular energy state will vary exponentially as the energy divided by \[{k_B}T\].
Where \[{k_B}T\] is the Boltzmann constant.
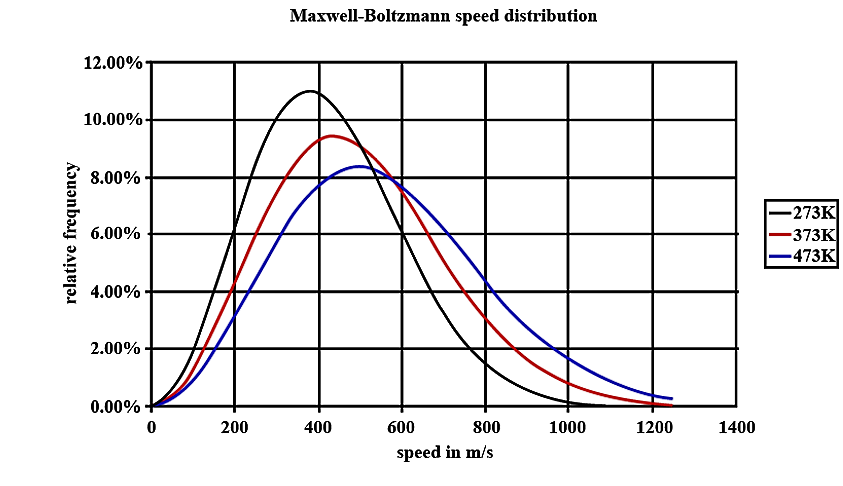
- In the graph given below there are three lines. These lines are drawn for the same number of particles but for different temperatures. For now, concentrate on the one marked 273 K. You should notice that there are some of the particles with low speeds, few with high speeds and many with medium speeds. The area that is present below the line will give the total number of particles in the gas. Now look at the 373K line, the total number of particles is the same so the total area under the line is the same, but more particles have faster speeds. The line has to move lower as the area underneath it can't change.
- We have to notice that at a very high speed, the area under the line will be very dependent on the temperature. For most reactions, the number of these molecules increases very rapidly as the temperature is raised. When there is a small increase in temperature, it can cause a larger increase of the number of particles present.
Therefore, the correct answer is option (B) The point A on x-axis represents the most probable speed and (C) The area under the curve gives the total number of molecules
So, the correct answer is “Option B”.
Note: The speed that is possessed by any type of molecule in the system corresponds to the maximum value or mode of f(v), that speed is the most probable speed. The distribution does not reflect any symmetricity.
Complete Solution :
Let us see what a Boltzmann distribution law is.
The motion of molecules is extremely chaotic. One individual molecule will be colliding with another molecule at an enormous rate. The rate of the collision is a billion times per second. Let us introduce the term \[{\rm{nv(E)}}\] which is the number density. This is also known as the distribution function. \[dE\]is the number of molecules that are present per unit volume whose energy is between \[E\] and \[E + dE\].
Based on the statistical mechanics, we can give :
\[{\rm{nv(E) = }}{{\rm{n}}_0}{e^{\frac{{ - E}}{{{k_B}T}}}}\]
According to the Boltzmann Law, the probability of finding a molecule at a particular energy state will vary exponentially as the energy divided by \[{k_B}T\].
Where \[{k_B}T\] is the Boltzmann constant.
- In the graph given below there are three lines. These lines are drawn for the same number of particles but for different temperatures. For now, concentrate on the one marked 273 K. You should notice that there are some of the particles with low speeds, few with high speeds and many with medium speeds. The area that is present below the line will give the total number of particles in the gas. Now look at the 373K line, the total number of particles is the same so the total area under the line is the same, but more particles have faster speeds. The line has to move lower as the area underneath it can't change.
- We have to notice that at a very high speed, the area under the line will be very dependent on the temperature. For most reactions, the number of these molecules increases very rapidly as the temperature is raised. When there is a small increase in temperature, it can cause a larger increase of the number of particles present.

Therefore, the correct answer is option (B) The point A on x-axis represents the most probable speed and (C) The area under the curve gives the total number of molecules
So, the correct answer is “Option B”.
Note: The speed that is possessed by any type of molecule in the system corresponds to the maximum value or mode of f(v), that speed is the most probable speed. The distribution does not reflect any symmetricity.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




