
The maximum number of isomers that can result from mono-bromination of 2-Methyl-2-pentene with N-bromosuccinimide in boiling ${ CCl }_{ 4 }$ is:
(a) 1
(b) 2
(c) 3
(d) 4
Answer
585k+ views
Hint: Isomers are compounds that have the same molecular formula (same number of atoms of each element) but have different arrangement of the atoms in three dimensional space. N-bromosuccinimide is used for allylic or benzylic bromination.
Complete step by step answer:
N-bromosuccinimide is a white crystalline solid and is used for allylic bromination.
In allylic bromination a hydrogen atom which is bonded to a carbon next to a double bond/aromatic ring is replaced by a bromine atom.
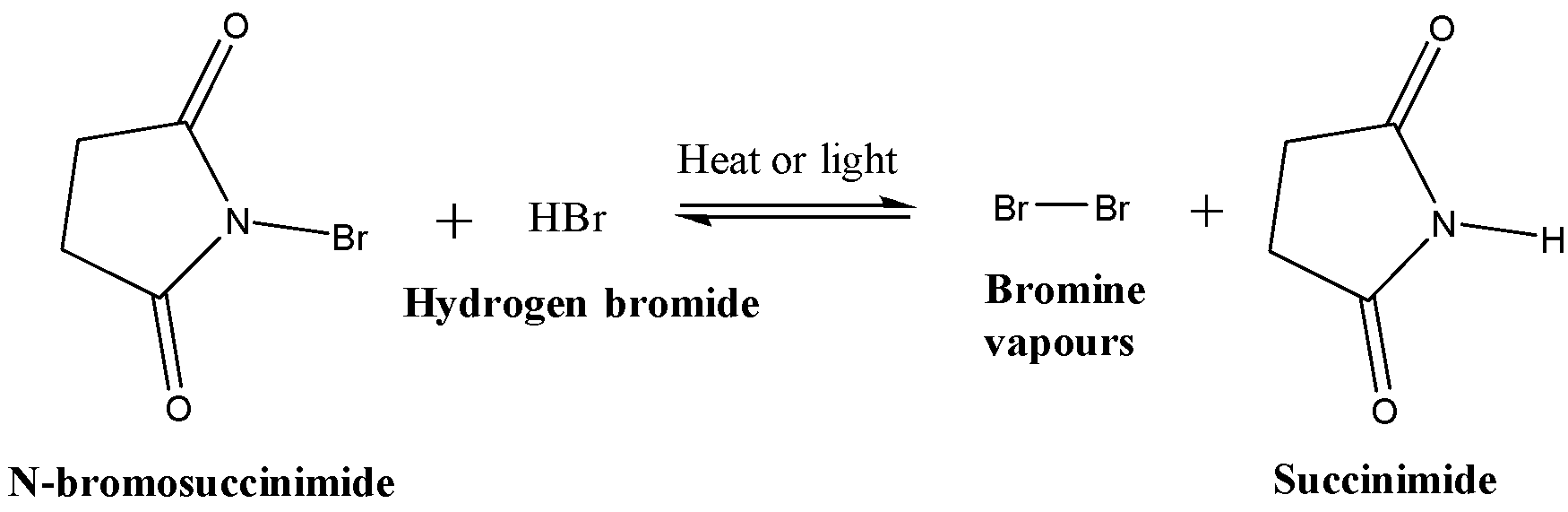
N-bromosuccinimide provides a constant low concentration of bromine vapours. The reaction is catalysed by HBr (trace amounts of HBr are generally present in N-bromosuccinimide). The reaction is reversible:
After the formation of the bromine vapours, bromine radicals are formed.
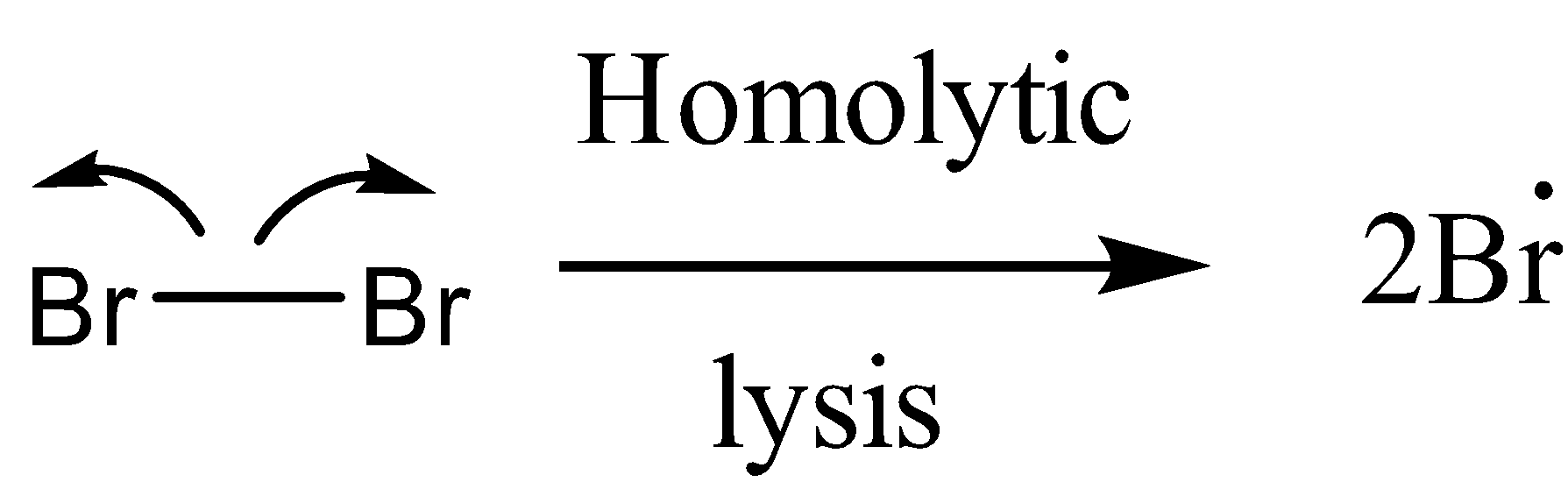
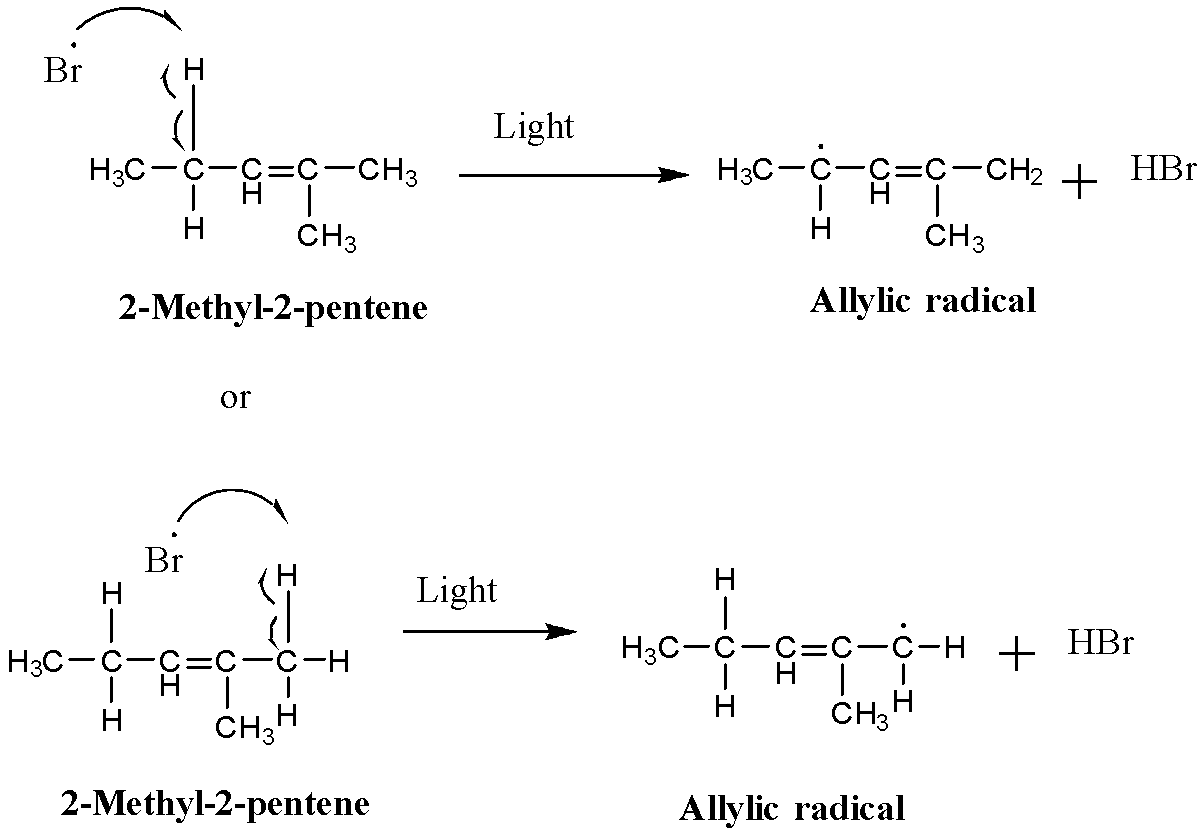
The bromine radical will abstract the hydrogen radical from the allylic carbon.
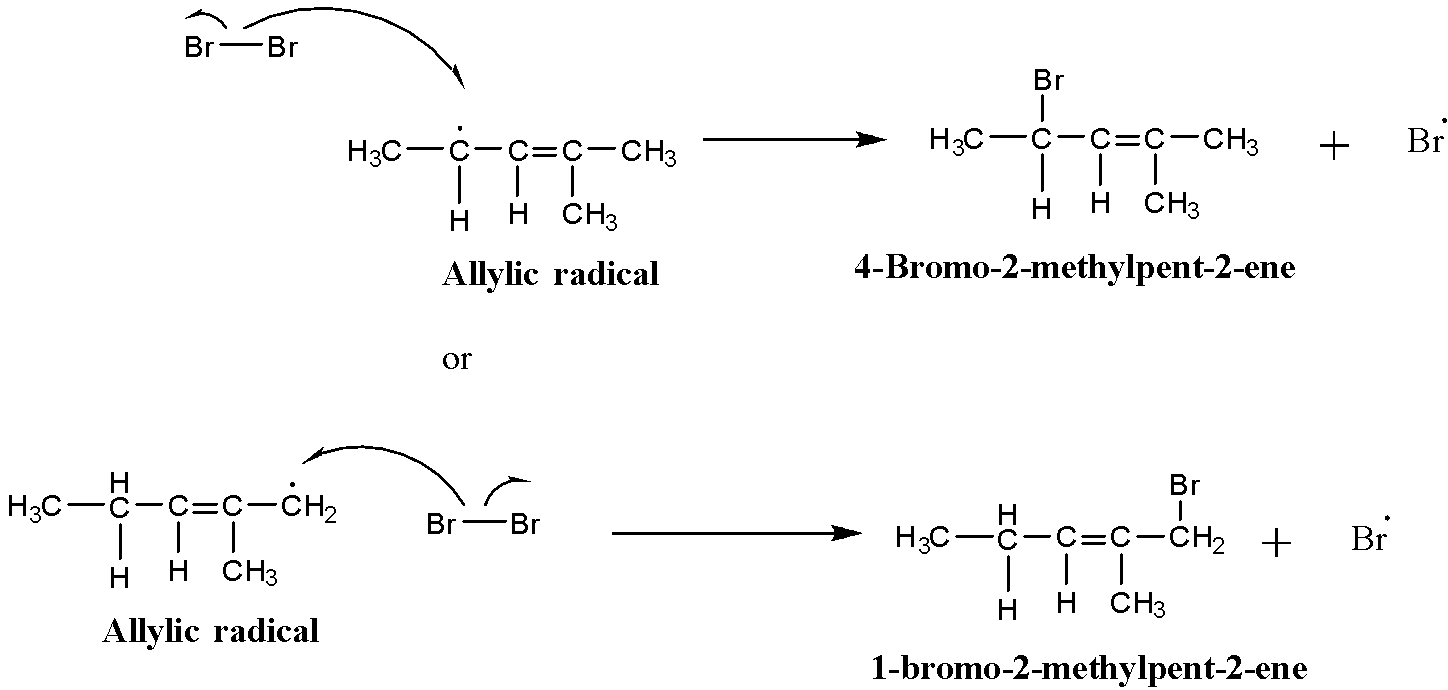
The allylic radical thus formed reacts with the bromine vapours to give the mono-brominated product:
Hence the reactions are:
$\begin{matrix} { CH }_{ 3 }-{ CH }_{ 2 }-CH=C({ CH }_{ 3 })-{ CH }_{ 3 } \\ 2-Methyl-2-pentene \end{matrix}\xrightarrow [ N-bromosuccinimide ]{ Boiling\quad { CCl }_{ 4 } } \begin{matrix} { CH }_{ 3 }-{ CH(Br)-CH=C{ (CH }_{ 3 })-{ CH }_{ 3 } } \\ 4-Bromo-2-methylpent-2-ene \end{matrix}$
$\begin{matrix} { CH }_{ 3 }-{ CH }_{ 2 }-CH=C({ CH }_{ 3 })-{ CH }_{ 3 } \\ 2-Methyl-2-pentene \end{matrix}\xrightarrow [ N-bromosuccinimide ]{ Boiling\quad { CCl }_{ 4 } } \begin{matrix} { CH }_{ 3 }-{ { CH }_{ 2 }-CH=C{ (CH }_{ 3 })-{ CH }_{ 2 }(Br) } \\ 1-Bromo-2-methylpent-2-ene \end{matrix}$
The process keeps on repeating until all of the N-bromosuccinimide is consumed.
Hence, two isomers are possible. Now in 4-Bromo-2-methylpent-2-ene, the carbon that is brominated is a chiral center, due to which two stereoisomers are possible for this structure and they will be enantiomers of each other.
Similarly in 1-Bromo-2-methylpent-2-ene, two geometrical isomers are possible. The E and Z isomers are shown below:
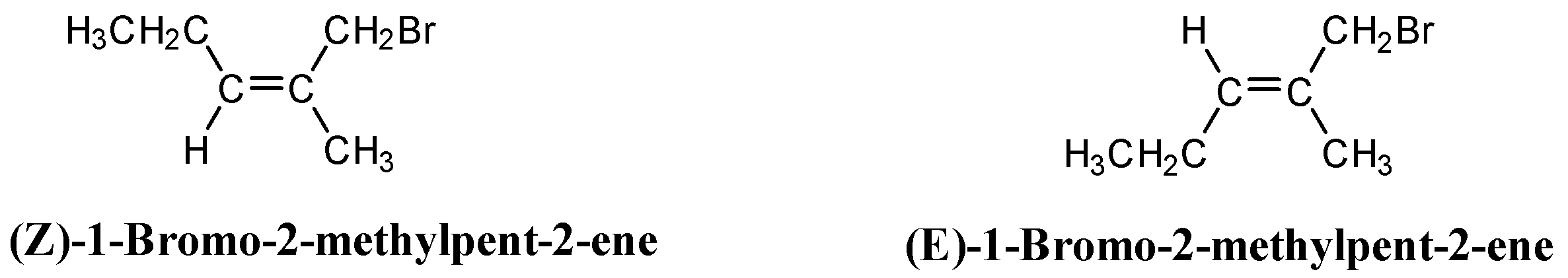
So, a total of four isomers are formed.
Hence the correct answer is (d) 4.
Note: 4-Bromo-2-methylpent-2-ene does not show geometrical isomerism. This is because one of the carbons that is doubly bonded have the same groups (methyl groups) attached to it.
Complete step by step answer:
N-bromosuccinimide is a white crystalline solid and is used for allylic bromination.
In allylic bromination a hydrogen atom which is bonded to a carbon next to a double bond/aromatic ring is replaced by a bromine atom.
N-bromosuccinimide provides a constant low concentration of bromine vapours. The reaction is catalysed by HBr (trace amounts of HBr are generally present in N-bromosuccinimide). The reaction is reversible:

After the formation of the bromine vapours, bromine radicals are formed.
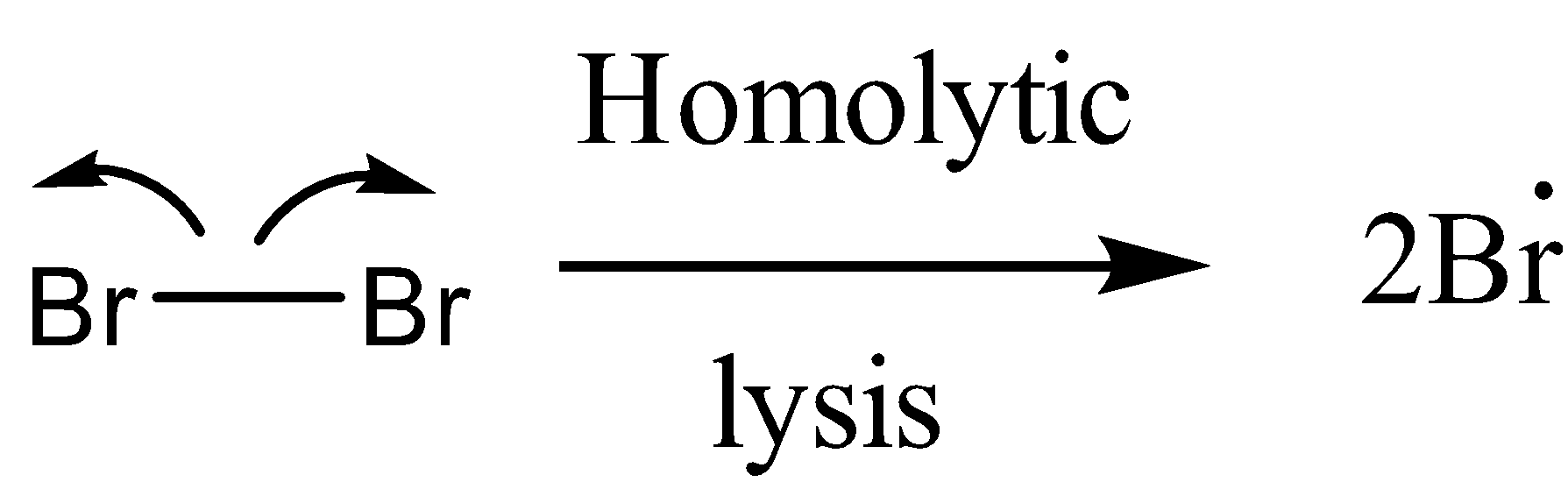
The bromine radical will abstract the hydrogen radical from the allylic carbon.

The allylic radical thus formed reacts with the bromine vapours to give the mono-brominated product:

Hence the reactions are:
$\begin{matrix} { CH }_{ 3 }-{ CH }_{ 2 }-CH=C({ CH }_{ 3 })-{ CH }_{ 3 } \\ 2-Methyl-2-pentene \end{matrix}\xrightarrow [ N-bromosuccinimide ]{ Boiling\quad { CCl }_{ 4 } } \begin{matrix} { CH }_{ 3 }-{ CH(Br)-CH=C{ (CH }_{ 3 })-{ CH }_{ 3 } } \\ 4-Bromo-2-methylpent-2-ene \end{matrix}$
$\begin{matrix} { CH }_{ 3 }-{ CH }_{ 2 }-CH=C({ CH }_{ 3 })-{ CH }_{ 3 } \\ 2-Methyl-2-pentene \end{matrix}\xrightarrow [ N-bromosuccinimide ]{ Boiling\quad { CCl }_{ 4 } } \begin{matrix} { CH }_{ 3 }-{ { CH }_{ 2 }-CH=C{ (CH }_{ 3 })-{ CH }_{ 2 }(Br) } \\ 1-Bromo-2-methylpent-2-ene \end{matrix}$
The process keeps on repeating until all of the N-bromosuccinimide is consumed.
Hence, two isomers are possible. Now in 4-Bromo-2-methylpent-2-ene, the carbon that is brominated is a chiral center, due to which two stereoisomers are possible for this structure and they will be enantiomers of each other.
Similarly in 1-Bromo-2-methylpent-2-ene, two geometrical isomers are possible. The E and Z isomers are shown below:
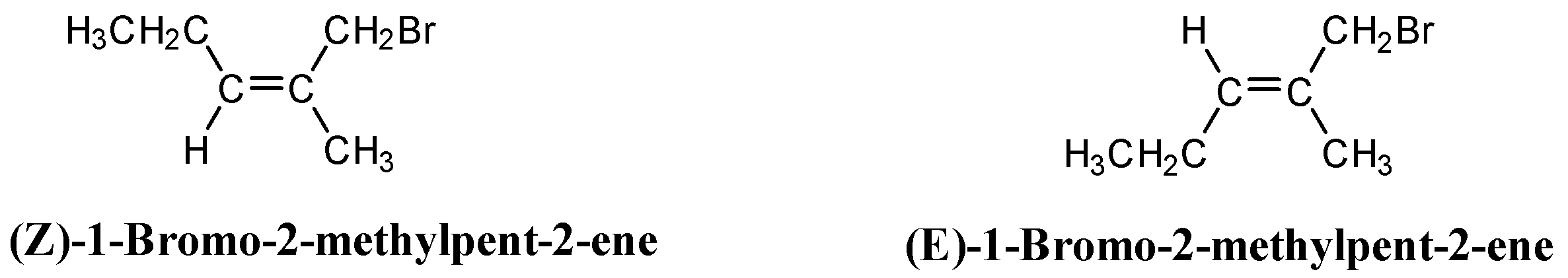
So, a total of four isomers are formed.
Hence the correct answer is (d) 4.
Note: 4-Bromo-2-methylpent-2-ene does not show geometrical isomerism. This is because one of the carbons that is doubly bonded have the same groups (methyl groups) attached to it.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a labelled diagram of the human heart and label class 11 biology CBSE

What is 1s 2s 2p 3s 3p class 11 chemistry CBSE




