
The major products P and Q are:
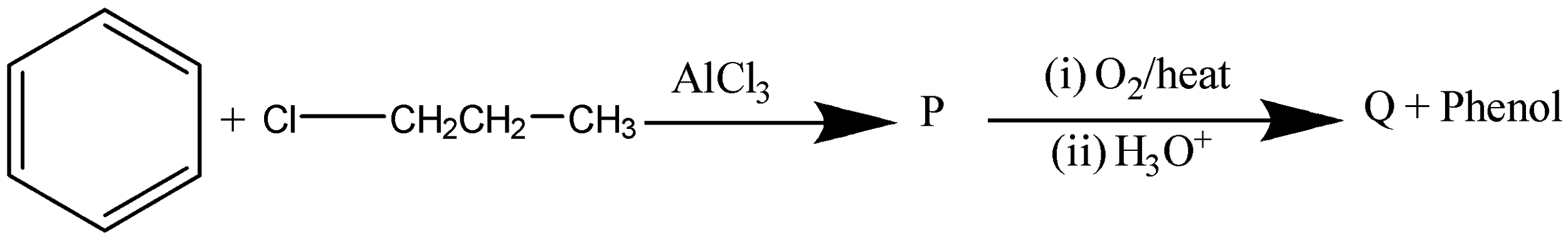
A.
 and $C{H_3}C{H_2}CHO$
and $C{H_3}C{H_2}CHO$
B.
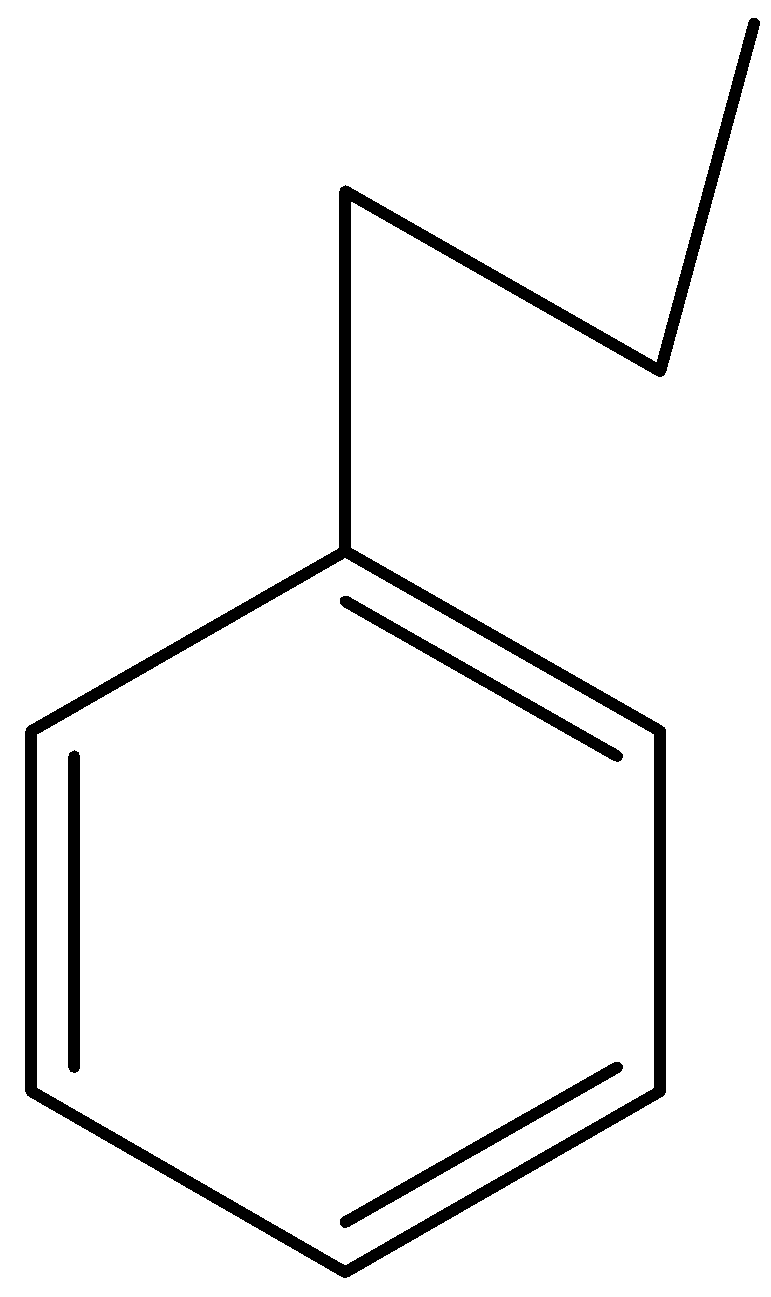
 and $C{H_3}COC{H_3}$
and $C{H_3}COC{H_3}$
C.
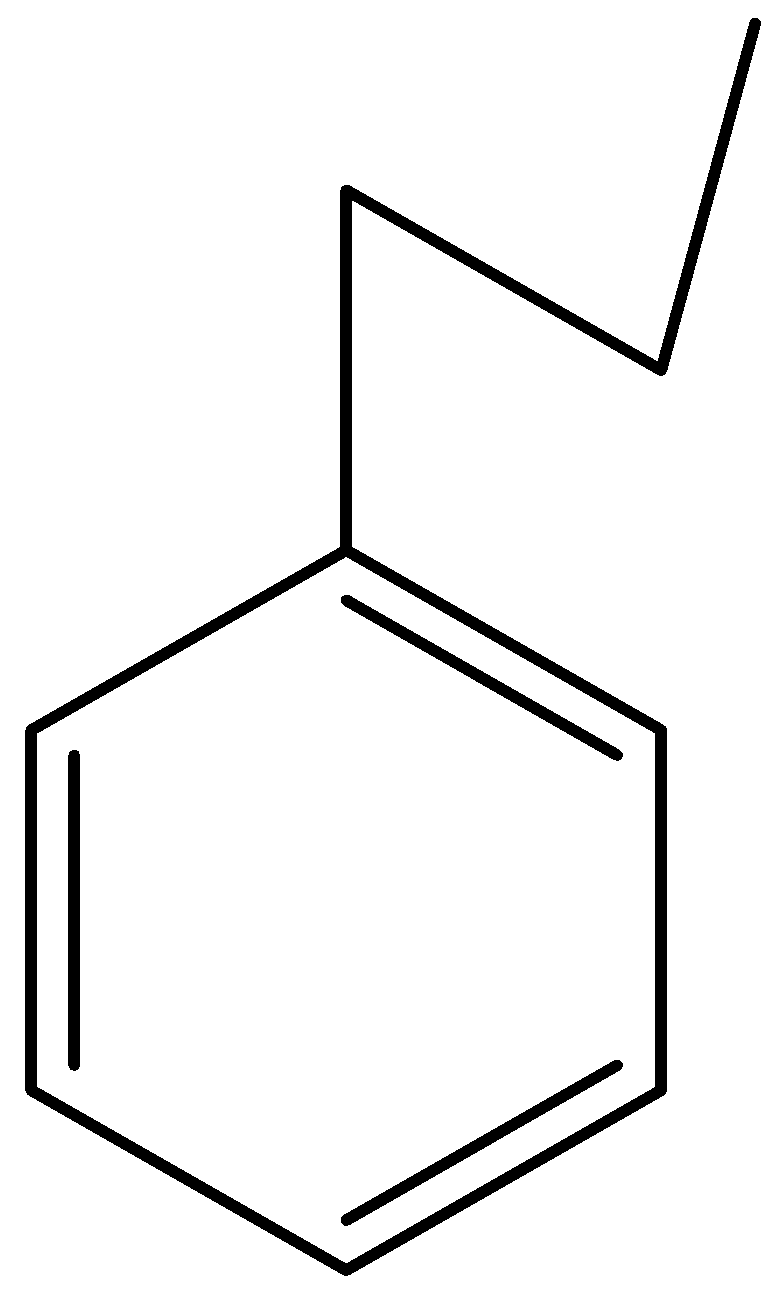
 and $C{H_3}COC{H_3}$
and $C{H_3}COC{H_3}$
D.
 and \[C{H_3}C{H_2}CHO\]
and \[C{H_3}C{H_2}CHO\]





Answer
566.7k+ views
Hint: This is a simple reaction between the given reaction. We will follow the given instructions and process ahead to determine the components. The reaction takes place in 2 stages. In the first stage the given compound benzene is reacted with propyl chloride in the presence of aluminium chloride. This reaction will yield Isopropylbenzene, also called cumene. Then this cumene is further reacted with oxygen, followed by hydrolysis it will yield a phenol and acetone.
Complete answer:
Let us process the reaction step-by-step.
In the first stage, benzene is reacted with propyl chloride in the presence of aluminium chloride.
The reaction takes place as follows:

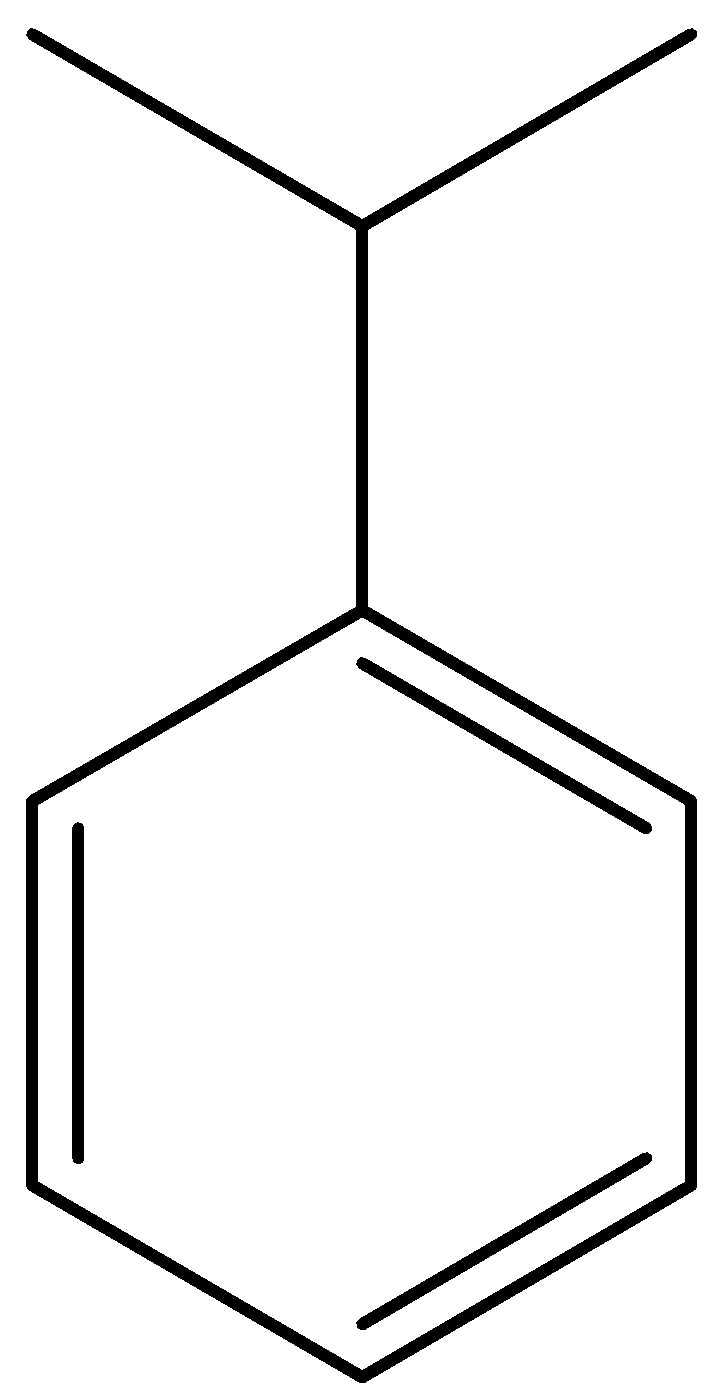
Here, isopropylbenzene is formed, which is also called as cumene. This is our ‘P’ compound.
Now, in the further stage of reaction, first the cumene is reacted with oxygen in the presence of heat and a complex is formed.
The reaction takes place as follows:
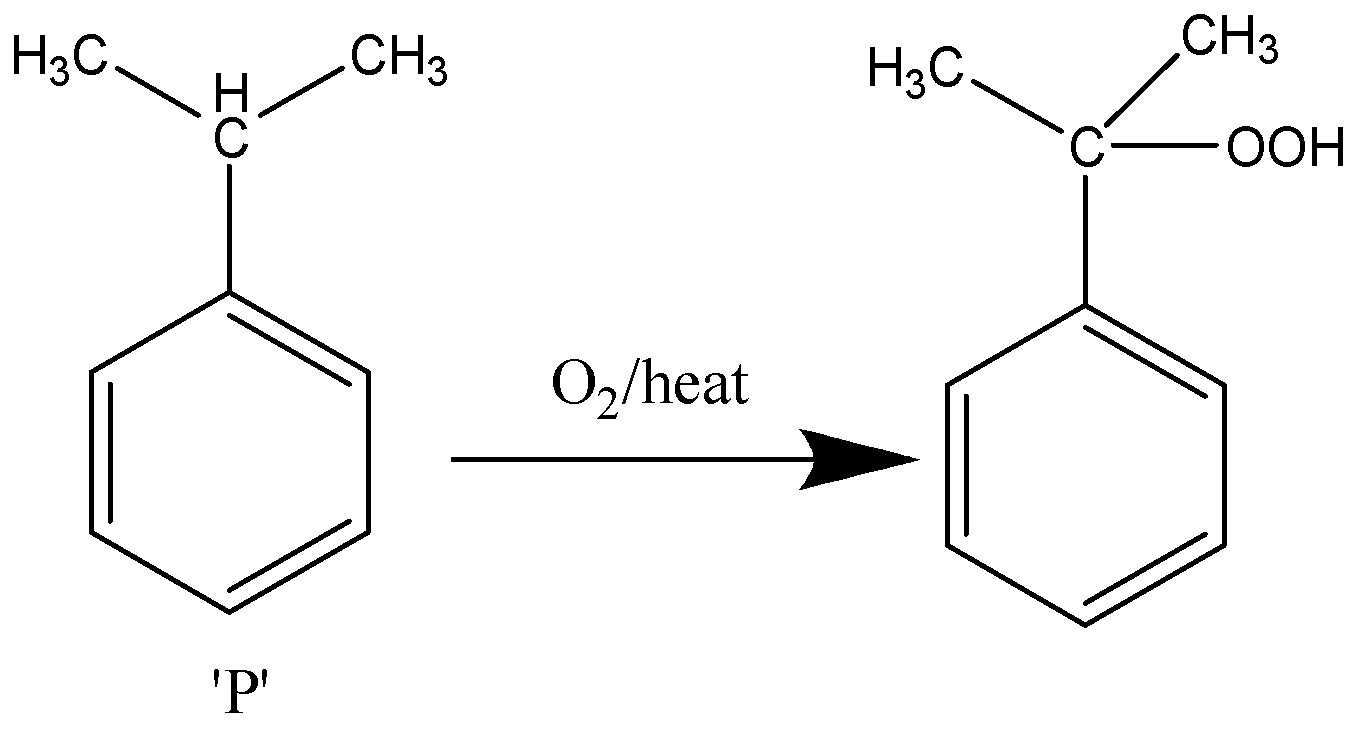
The complex undergoes hydrolysis as defined in the problem.
The reaction takes place as follows
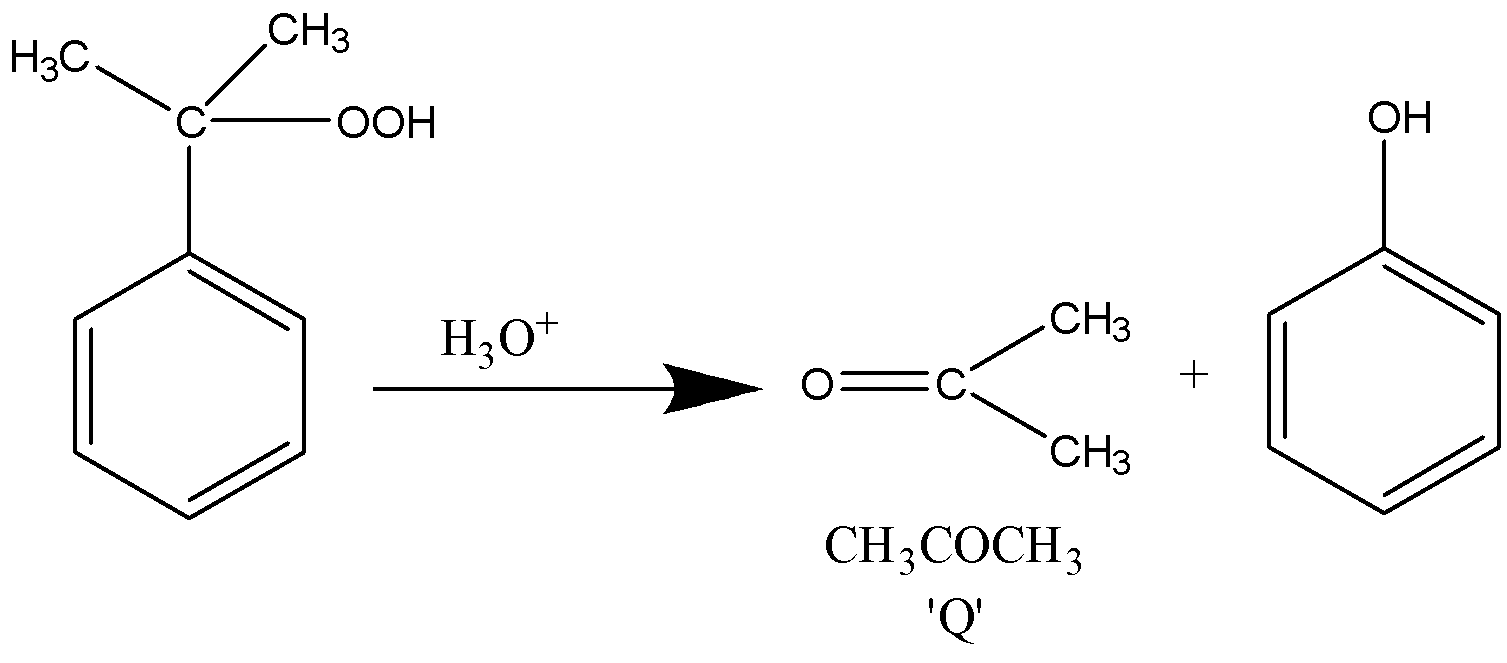
The resultant product formed is phenol along with acetone which is our product ‘Q’, chemically written as \[C{H_3}COC{H_3}\] .
Hence, the correct answer is option (C).
Note:
When a benzene is reacted with alkyl halide, it forms iso-benzyl alkane in the presence of aluminium chloride. And this is-benzyl alkane compound when reacted with oxygen and undergoes the process of hydrolysis, it yields acetone and phenol (alcohol). This type of reaction is used to yield acetone and phenols.
Complete answer:
Let us process the reaction step-by-step.
In the first stage, benzene is reacted with propyl chloride in the presence of aluminium chloride.
The reaction takes place as follows:

Here, isopropylbenzene is formed, which is also called as cumene. This is our ‘P’ compound.
Now, in the further stage of reaction, first the cumene is reacted with oxygen in the presence of heat and a complex is formed.
The reaction takes place as follows:

The complex undergoes hydrolysis as defined in the problem.
The reaction takes place as follows

The resultant product formed is phenol along with acetone which is our product ‘Q’, chemically written as \[C{H_3}COC{H_3}\] .
Hence, the correct answer is option (C).
Note:
When a benzene is reacted with alkyl halide, it forms iso-benzyl alkane in the presence of aluminium chloride. And this is-benzyl alkane compound when reacted with oxygen and undergoes the process of hydrolysis, it yields acetone and phenol (alcohol). This type of reaction is used to yield acetone and phenols.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




