
What will be the major product, which can be obtained from the following reaction?
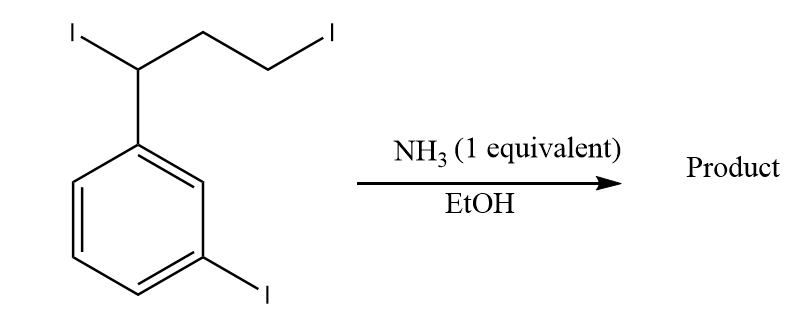
1)
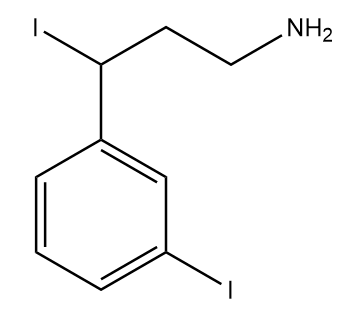
2)
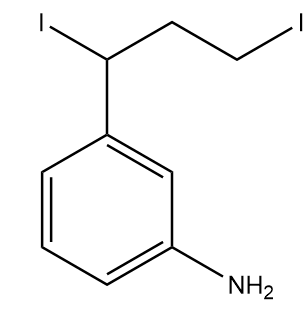
3)
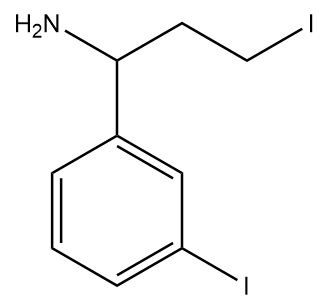
4)
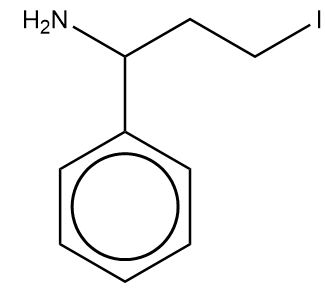





Answer
515.4k+ views
Hint: Nucleophilic substitution reaction: It is a type of an organic reaction in which a nucleophile replaces a nucleophile present in the reactant. The nucleophile which leaves the reactant after reaction is known as the leaving group. Greater the size of the leaving group, more easily it can be replaced by a nucleophile.
Complete answer:
Bimolecular nucleophilic substitution reaction: It is an organic reaction in which attack of nucleophile and removal of leaving group takes place in a single step but the overall reaction depends on both reactant as well as nucleophile. No intermediate is formed in this reaction but it passes through a transition state. As the attack of the nucleophile takes place from the backside, the retention in configuration is observed in the product. Hence, these reactions are inherently stereoselective reactions.
For the given organic reaction, it follows $ S{{N}_{2}} $ reaction mechanism which is discussed as follows:
Step-1: Attack of lone pairs of ammonia to the primary carbon atom.
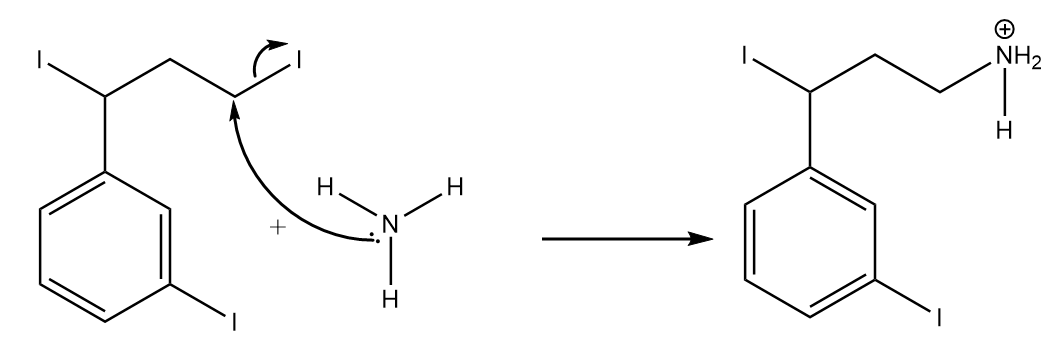
Step-2: Removal of $ {{H}^{+}} $ ion:
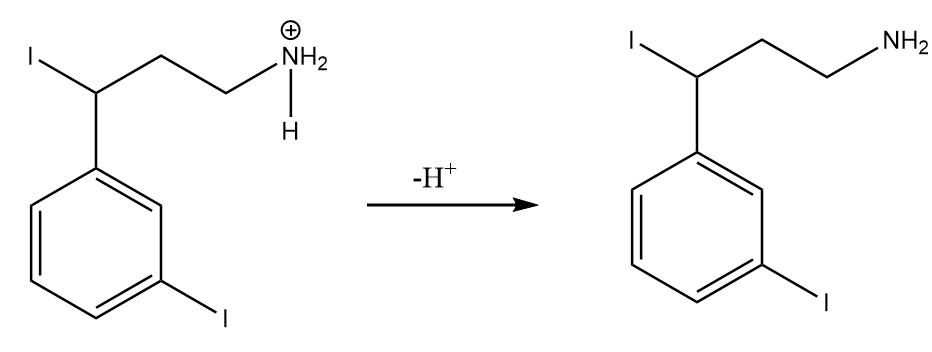
Hence, the final product formed after the reaction is as follows:
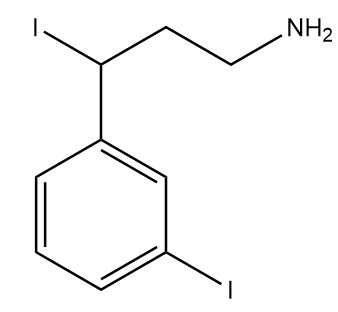
Thus, option (1) is the correct answer.
Note:
It is important to note that bimolecular nucleophilic substitution reactions i.e., $ S{{N}_{2}} $ reactions are concerted step reaction i.e., attack of nucleophile and removal of leaving group takes place simultaneously. Hence, less sterically hindered carbon is more preferred for $ S{{N}_{2}} $ reaction mechanism. The order of attack of nucleophile in $ S{{N}_{2}} $ reaction is $ {{1}^{\circ }}>{{2}^{\circ }}>{{3}^{\circ }} $ .
Complete answer:
Bimolecular nucleophilic substitution reaction: It is an organic reaction in which attack of nucleophile and removal of leaving group takes place in a single step but the overall reaction depends on both reactant as well as nucleophile. No intermediate is formed in this reaction but it passes through a transition state. As the attack of the nucleophile takes place from the backside, the retention in configuration is observed in the product. Hence, these reactions are inherently stereoselective reactions.
For the given organic reaction, it follows $ S{{N}_{2}} $ reaction mechanism which is discussed as follows:
Step-1: Attack of lone pairs of ammonia to the primary carbon atom.

Step-2: Removal of $ {{H}^{+}} $ ion:

Hence, the final product formed after the reaction is as follows:

Thus, option (1) is the correct answer.
Note:
It is important to note that bimolecular nucleophilic substitution reactions i.e., $ S{{N}_{2}} $ reactions are concerted step reaction i.e., attack of nucleophile and removal of leaving group takes place simultaneously. Hence, less sterically hindered carbon is more preferred for $ S{{N}_{2}} $ reaction mechanism. The order of attack of nucleophile in $ S{{N}_{2}} $ reaction is $ {{1}^{\circ }}>{{2}^{\circ }}>{{3}^{\circ }} $ .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




