
The major product of the given reaction is:
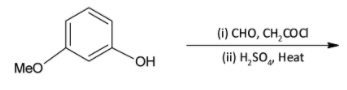
A.
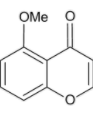
B.
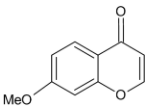
C.
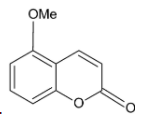
D.
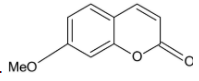





Answer
548.7k+ views
Hint:
To answer this question, you must be familiar with reactions of various oxygen containing organic compounds. Acid chlorides are much more reactive than aldehydes, ketones and carboxylic acids.
Complete step by step solution:
We know that acid chlorides are more reactive than aldehydes and thus the acid chloride group will be the one reacting with the phenolic hydroxyl group. The acid chloride and hydroxyl group undergoes an esterification reaction.
We are familiar with the fact that methoxy group is an ortho para directing group. Thus, we can say that the para position (to methoxy group) of the phenyl ring is activated and susceptible to nucleophilic attack.
On treatment with concentrated sulphuric acid, a water molecule is lost and the molecule undergoes cyclisation. A six membered cyclic ring is formed.
We can write the reaction as,
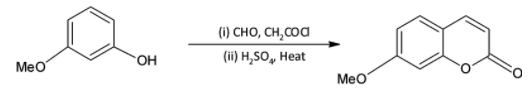
Thus, the correct option is D.
Note:
Acid halides are derived from carboxylic acids by replacing the hydroxyl group in the acid by a halide. A unique property of an acid halide is its inability to form hydrogen bonds with other compounds. This absence of hydrogen bonds affects their boiling and melting point. In comparison, it can be clearly seen that the melting and boiling points of acid halides are significantly lower than those of carboxylic acids.
Acid halides are extremely reactive compounds. Each of their reactions involves the replacement of the halide group by another functional group, that might be the reacting nucleophile. All reactions of acyl halides are characterized by production of steamy acidic fumes of hydrogen halides in the first step.
To answer this question, you must be familiar with reactions of various oxygen containing organic compounds. Acid chlorides are much more reactive than aldehydes, ketones and carboxylic acids.
Complete step by step solution:
We know that acid chlorides are more reactive than aldehydes and thus the acid chloride group will be the one reacting with the phenolic hydroxyl group. The acid chloride and hydroxyl group undergoes an esterification reaction.
We are familiar with the fact that methoxy group is an ortho para directing group. Thus, we can say that the para position (to methoxy group) of the phenyl ring is activated and susceptible to nucleophilic attack.
On treatment with concentrated sulphuric acid, a water molecule is lost and the molecule undergoes cyclisation. A six membered cyclic ring is formed.
We can write the reaction as,

Thus, the correct option is D.
Note:
Acid halides are derived from carboxylic acids by replacing the hydroxyl group in the acid by a halide. A unique property of an acid halide is its inability to form hydrogen bonds with other compounds. This absence of hydrogen bonds affects their boiling and melting point. In comparison, it can be clearly seen that the melting and boiling points of acid halides are significantly lower than those of carboxylic acids.
Acid halides are extremely reactive compounds. Each of their reactions involves the replacement of the halide group by another functional group, that might be the reacting nucleophile. All reactions of acyl halides are characterized by production of steamy acidic fumes of hydrogen halides in the first step.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




