
The major product of the following reaction:-
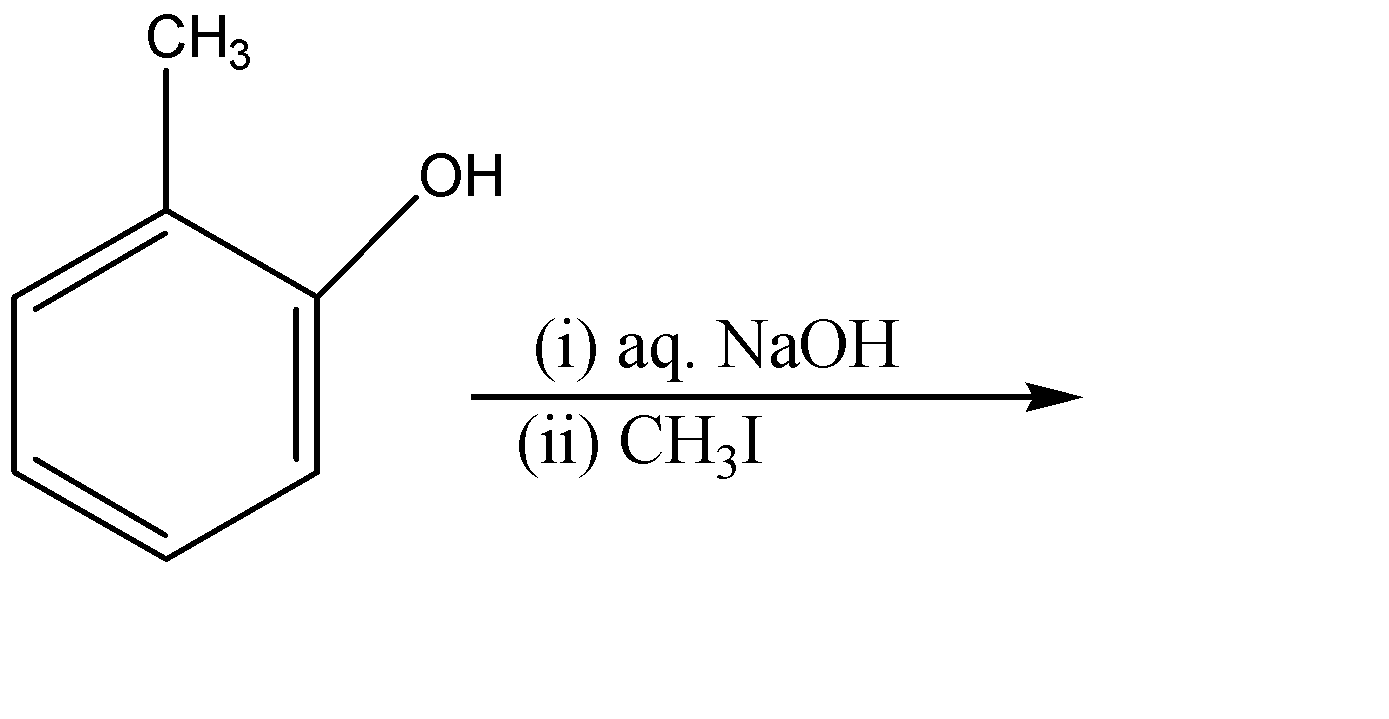
(A)
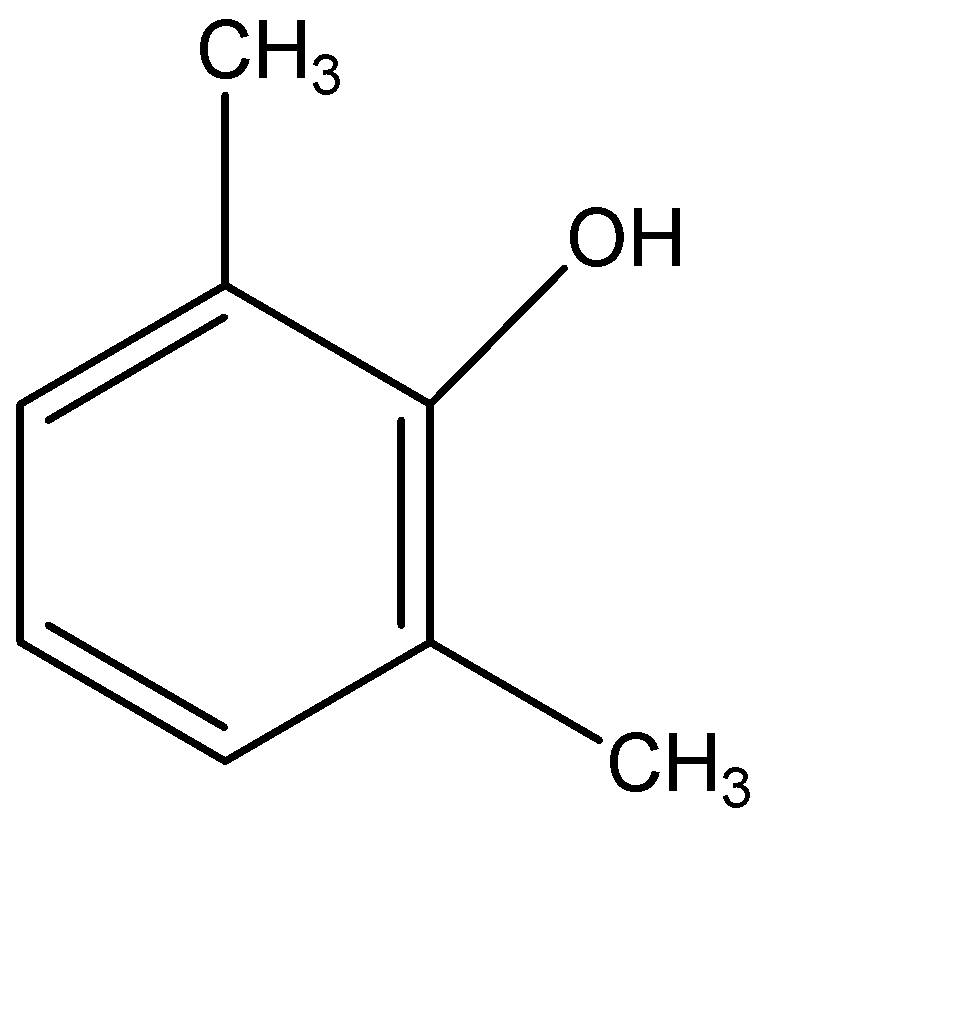
(B)
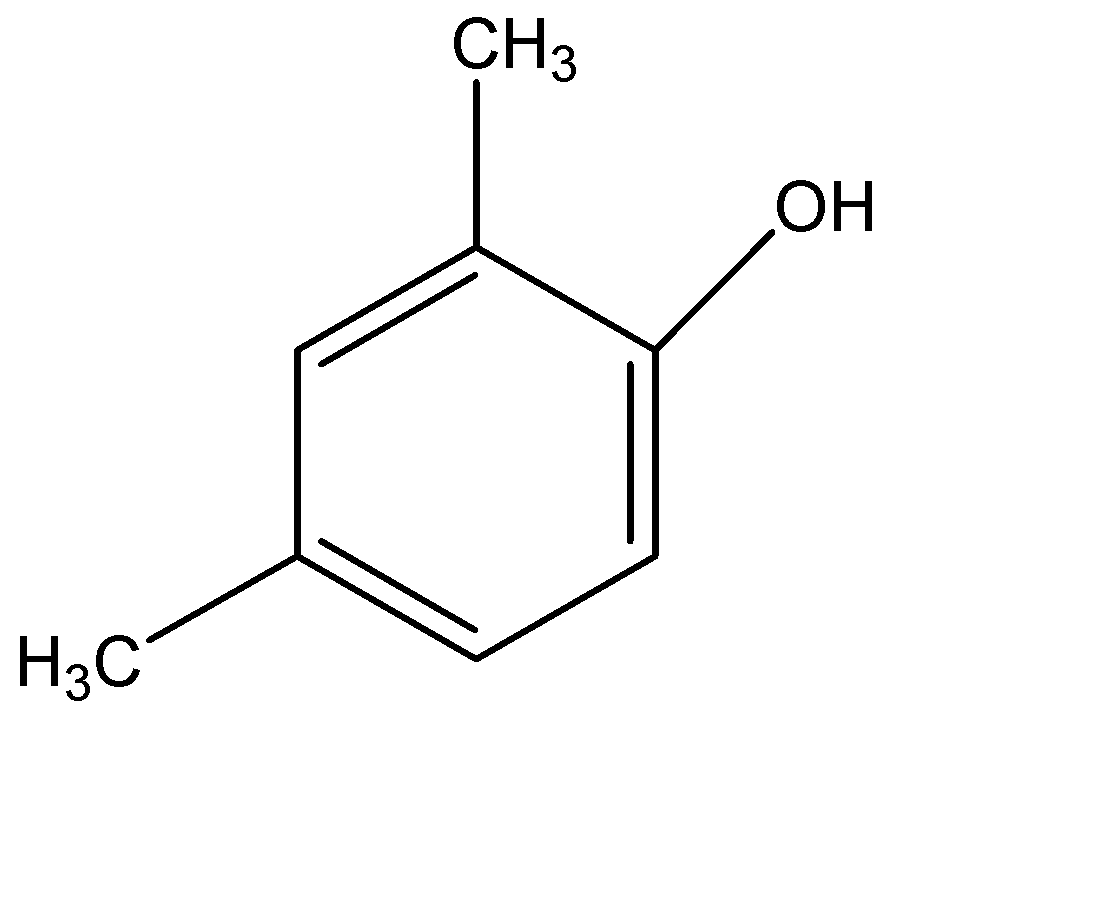
(C)
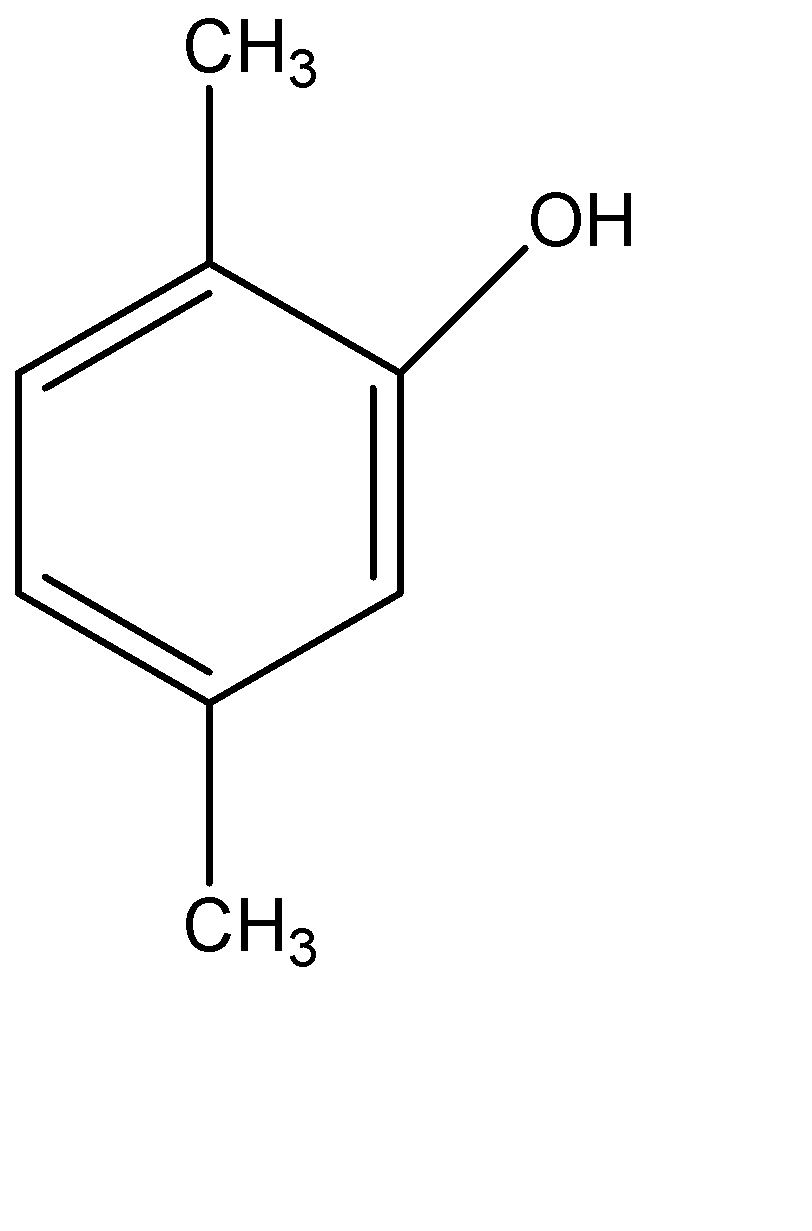
(D)
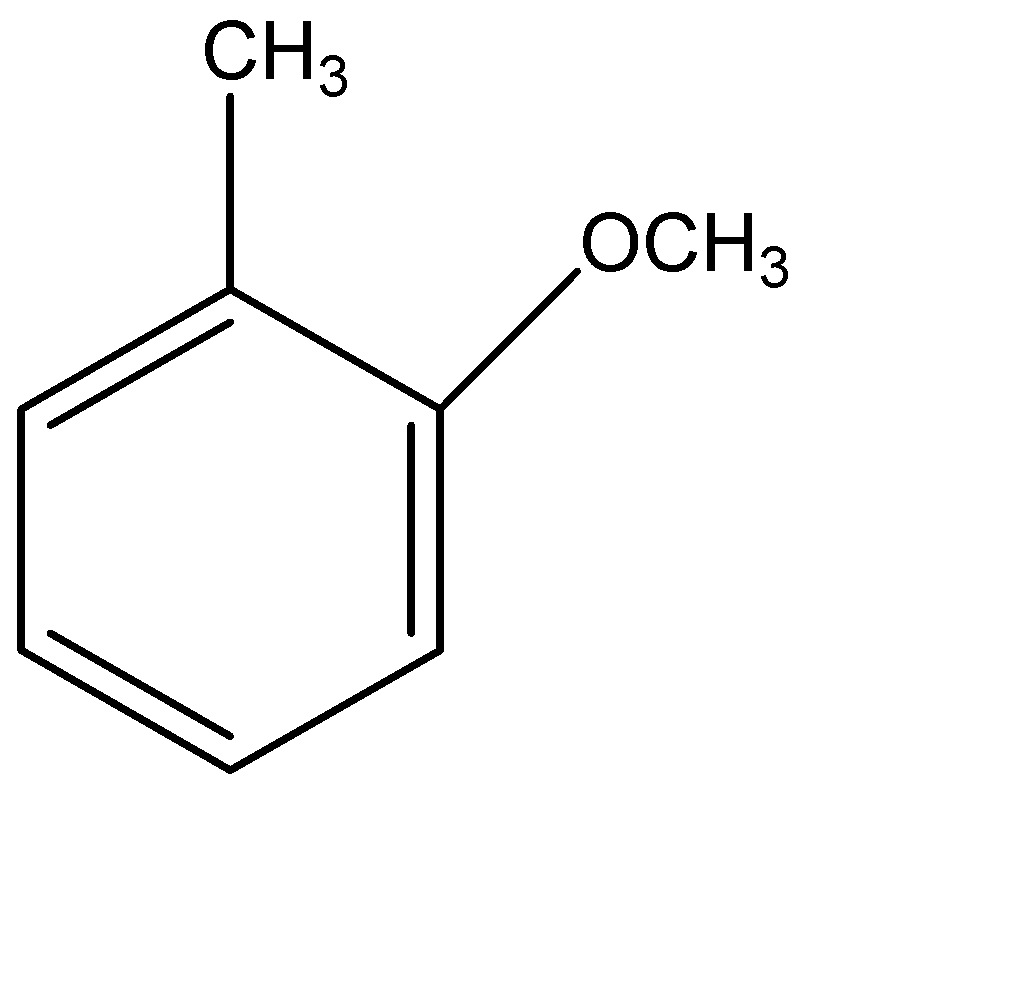





Answer
531k+ views
Hint: As we know that +M (mesomeric effect), +I (inductive effect) groups are mostly ortho - para directors and we must check which group is present, so that we can confirm the site of attack. Also the reagents provided in the reaction plays an important role.
Complete answer:
We are given 2-methyl phenol in the question. The alcohol ($-OH$) group attached to the ring can act as an acid as well as an ortho – para director group. So now we will check that which reagent is provided so as to confirm the way of reaction to be performed.
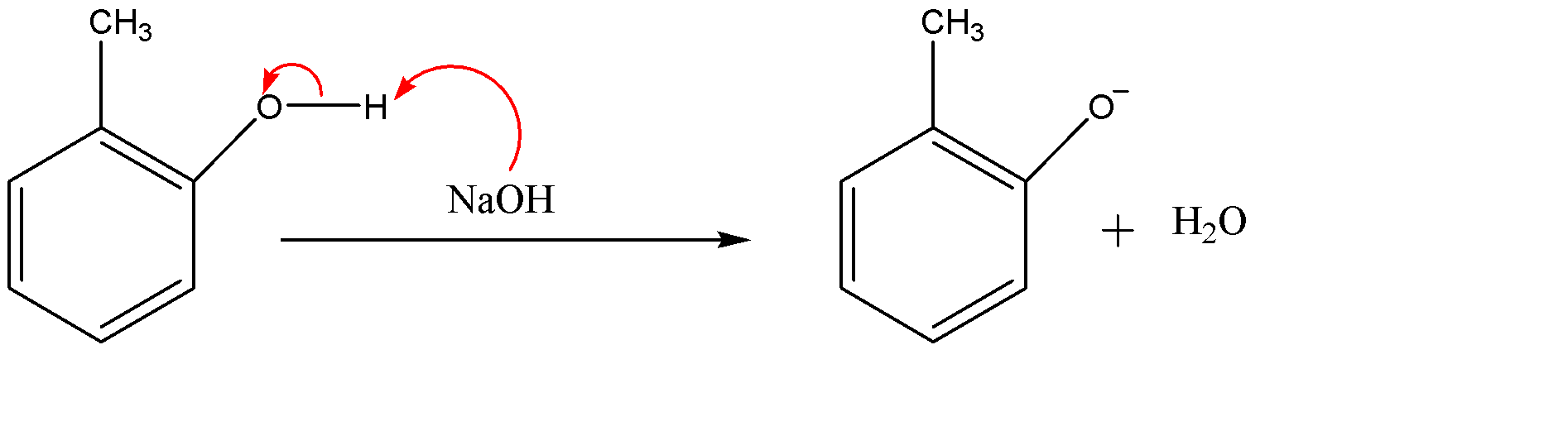
Since NaOH (sodium hydroxide) is given as the reagent which is a strong base. The hydroxide ($O{{H}^{-}}$) part of the base will attack on the acidic hydrogen of the alcohol group. And the electron cloud will shift on the oxygen atom. We will also get water molecules in this step.
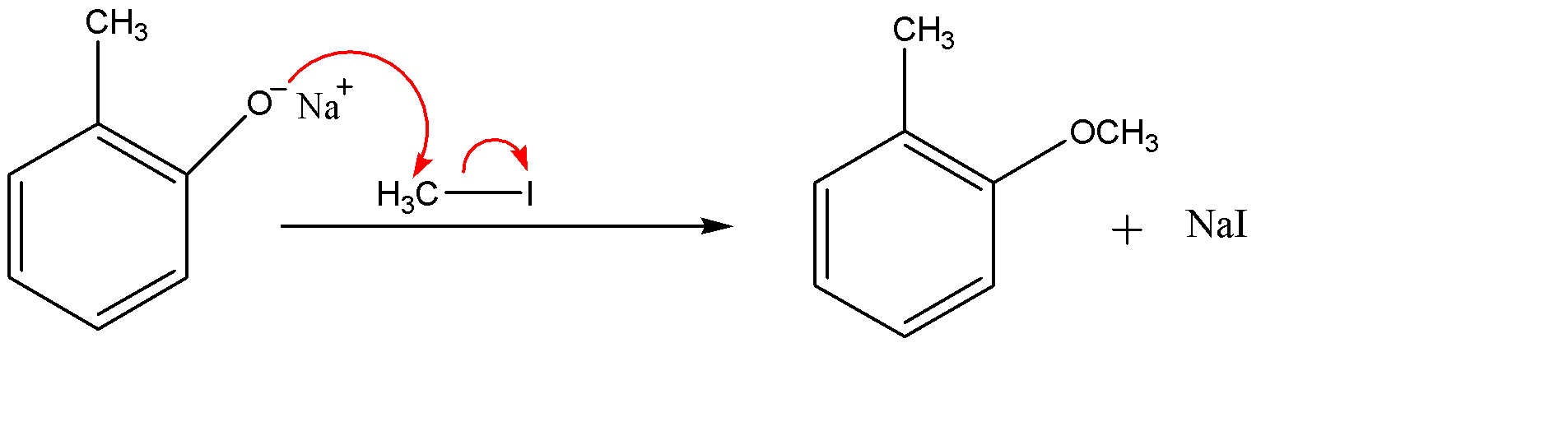
Since ${{O}^{-}}$ is a strong nucleophile whereas the methyl part of methyl iodide ($C{{H}_{3}}I$) act as an electrophile, therefore ${{O}^{-}}$ group of the compound will attack the methyl iodide and eliminate the iodide ion (${{I}^{-}}$). The iodide ion attracts the sodium ion and they form an ionic solid that is methyl iodide as a side product.
Therefore, the correct option is (D).
Note:
As it is known that acid-base reactions are the fastest reaction and hence in a solution, this reaction will always take place before all other reactions such as electrophilic, nucleophilic, substitution, addition or elimination reactions, etc.
Complete answer:
We are given 2-methyl phenol in the question. The alcohol ($-OH$) group attached to the ring can act as an acid as well as an ortho – para director group. So now we will check that which reagent is provided so as to confirm the way of reaction to be performed.

Since NaOH (sodium hydroxide) is given as the reagent which is a strong base. The hydroxide ($O{{H}^{-}}$) part of the base will attack on the acidic hydrogen of the alcohol group. And the electron cloud will shift on the oxygen atom. We will also get water molecules in this step.

Since ${{O}^{-}}$ is a strong nucleophile whereas the methyl part of methyl iodide ($C{{H}_{3}}I$) act as an electrophile, therefore ${{O}^{-}}$ group of the compound will attack the methyl iodide and eliminate the iodide ion (${{I}^{-}}$). The iodide ion attracts the sodium ion and they form an ionic solid that is methyl iodide as a side product.
Therefore, the correct option is (D).
Note:
As it is known that acid-base reactions are the fastest reaction and hence in a solution, this reaction will always take place before all other reactions such as electrophilic, nucleophilic, substitution, addition or elimination reactions, etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




