
The major product of the acetylation of salicylic acid with $A{c_2}\dfrac{O}{{{H^ + }}}$ followed by heating with anhydrous $AlC{l_3}$ is:
A.
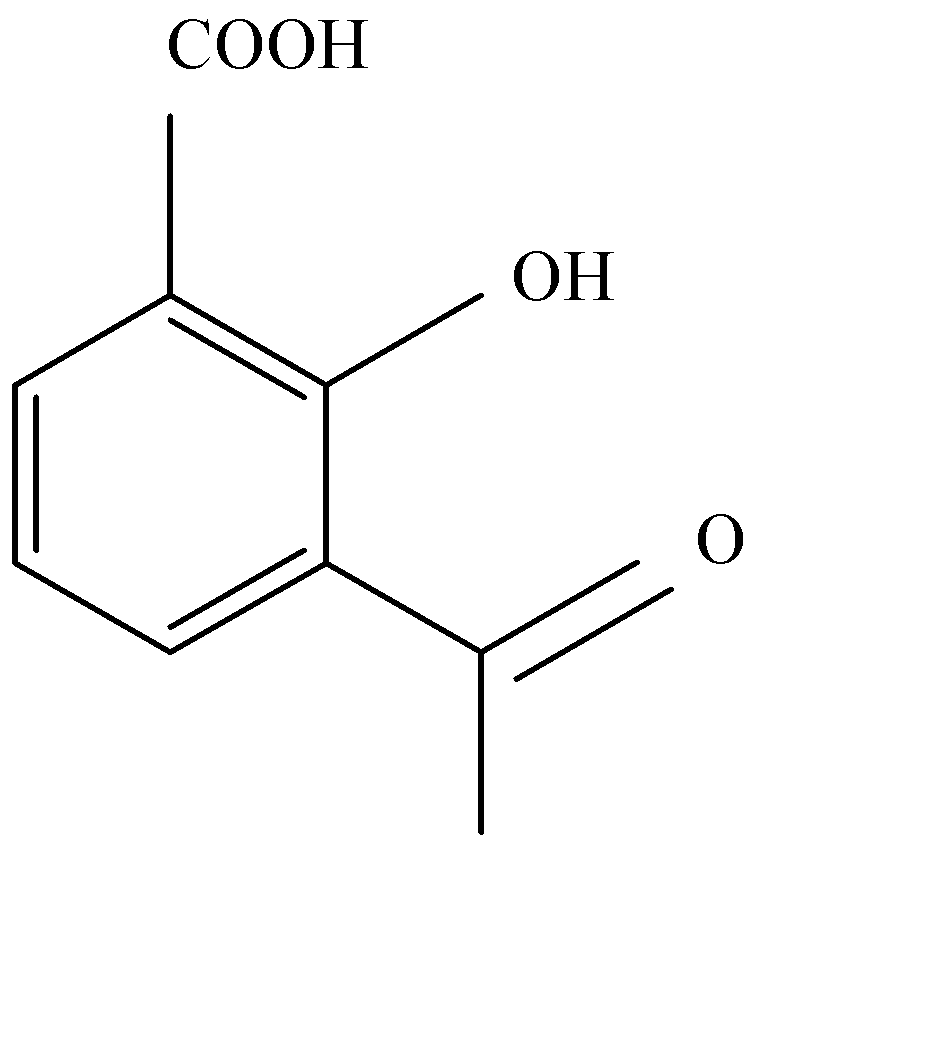
B.
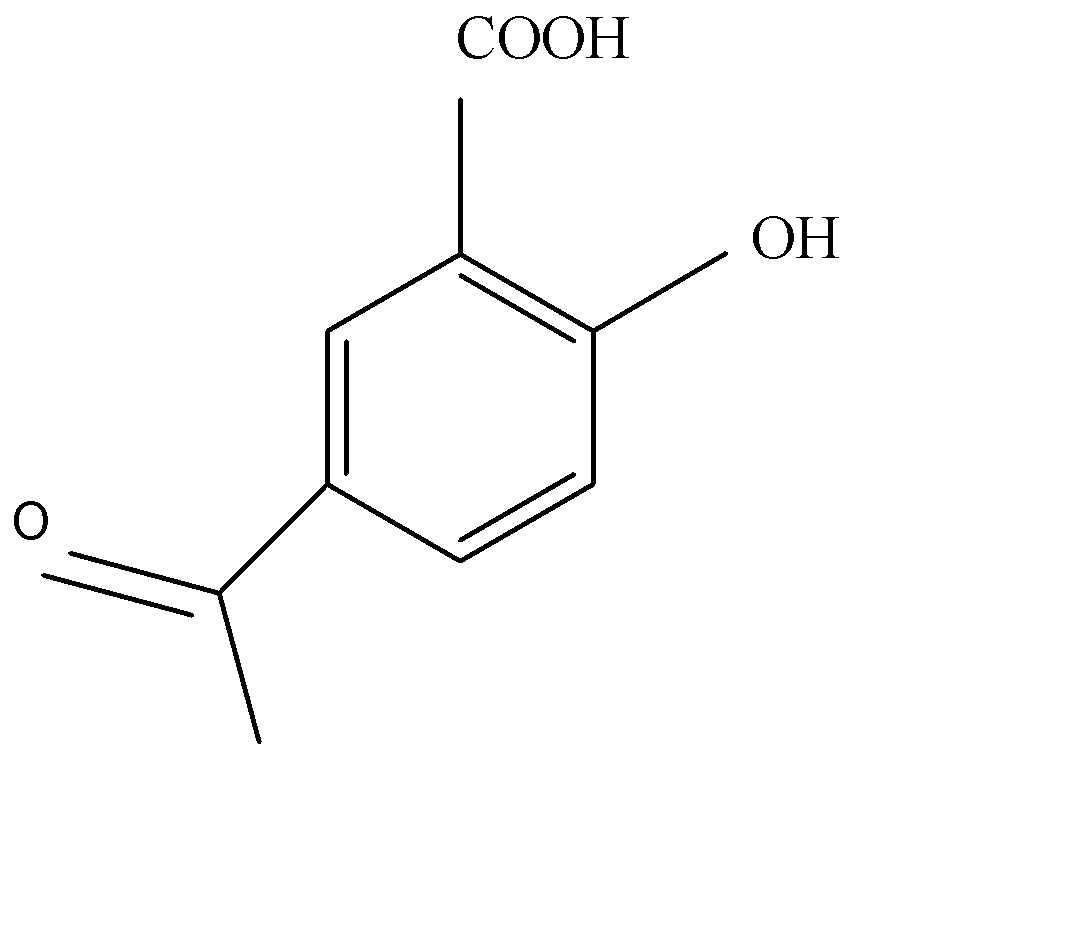
C.
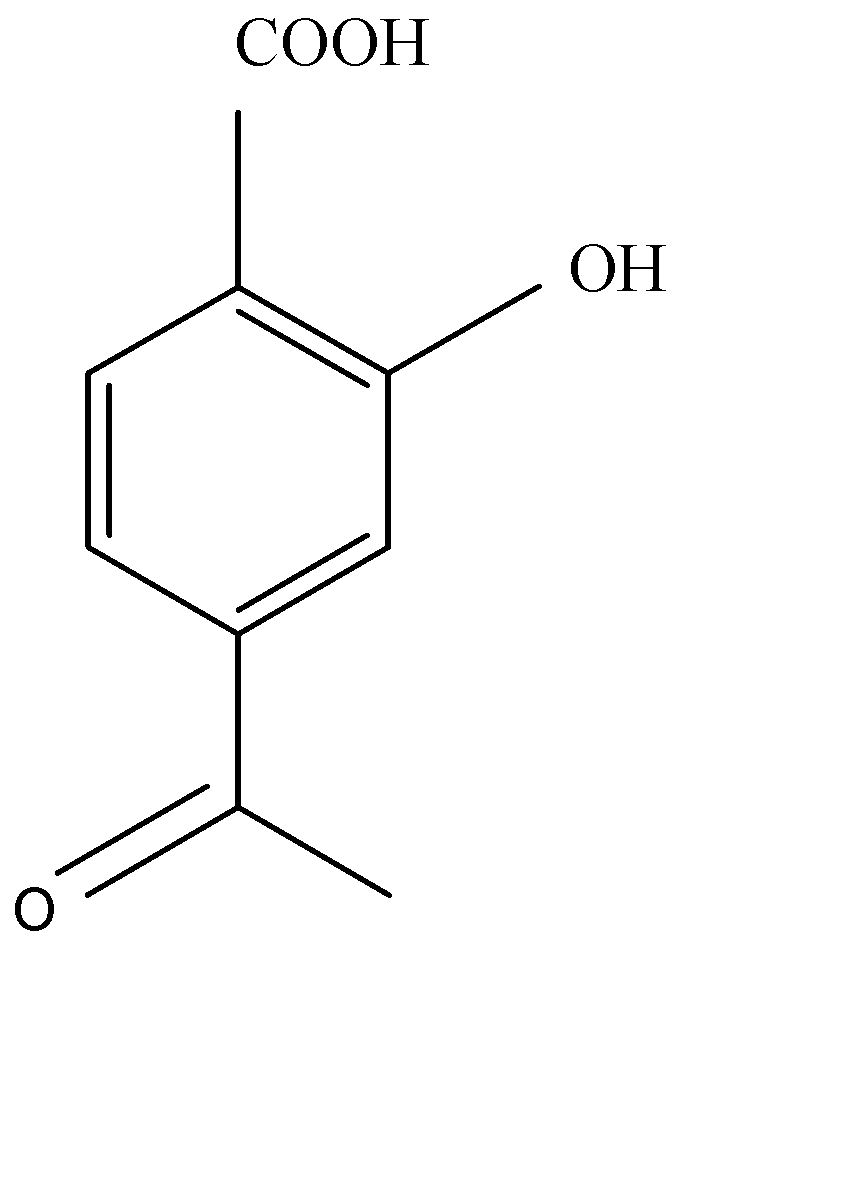
D.
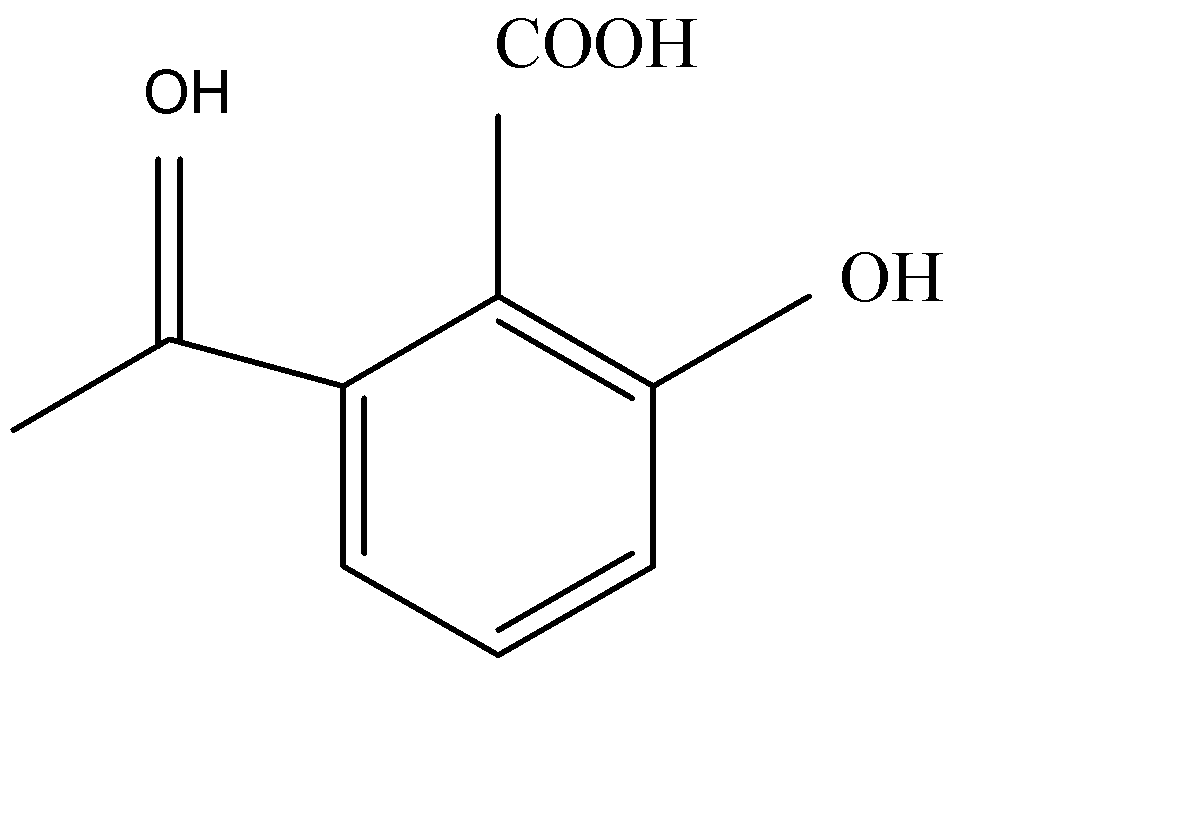




Answer
581.1k+ views
Hint:The acetylation of salicylic acid is an electrophilic reaction. This reaction occurs in the presence of a catalyst. This reaction is subjected to acetylation and the given product is acetic acid as the final product. An electron pair replaces the functional group. The three steps involved in the electrophilic substitution reaction. Acetylation introduced by acetyl group.
Complete step by step answer:
Acetylation of salicylic acid forms aspirin in acidic medium. Acetic anhydride interacts with salicylic acid in presence of conc. Sulphury acid for production of aspirin and given product of acetic acid. Acetylation of salicylic acid is electrophilic reaction. This reaction occurs in the presence of $A{c_2}O$ .
The synthesis of acetylation of salicylic acid is given below:
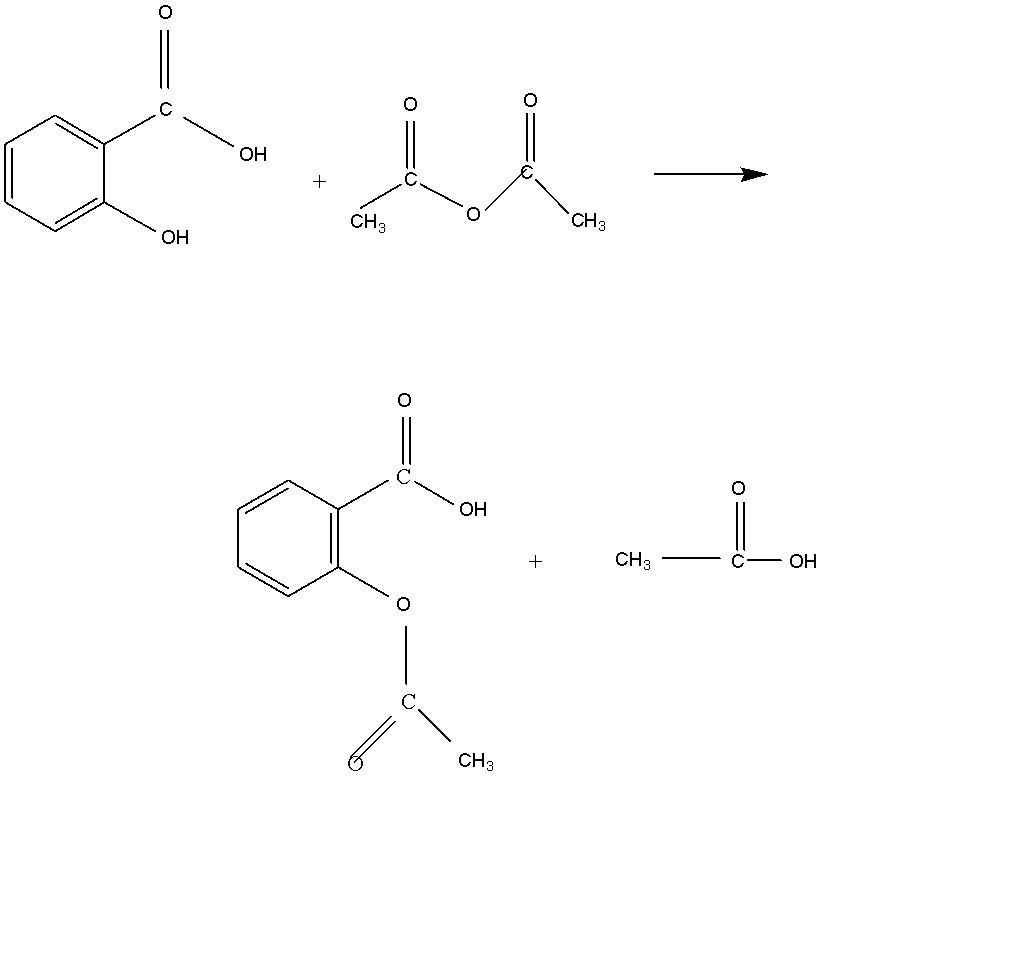
As we see the acetylation of salicylic acid gives us products of acetylsalicylic acid.
Therefore, Option (A) is the correct answer.
Additional information:
It is used for minor aches and mild to moderate pain which is recommended for arthritic conditions. It reduces risk of transient ischemic attacks in men. In organic chemistry, electrophile reaction in a chemical compound, the bond is broken and two new bonds are formed.
Note: In this reaction electron substitution reaction takes place. Here we remember how bond breaks and new bonds formed. The reaction takes place in the presence of a catalyst. Now we try to understand the formation of the product. There are acetic acid and aspirin given to us as products.
Complete step by step answer:
Acetylation of salicylic acid forms aspirin in acidic medium. Acetic anhydride interacts with salicylic acid in presence of conc. Sulphury acid for production of aspirin and given product of acetic acid. Acetylation of salicylic acid is electrophilic reaction. This reaction occurs in the presence of $A{c_2}O$ .
The synthesis of acetylation of salicylic acid is given below:

As we see the acetylation of salicylic acid gives us products of acetylsalicylic acid.
Therefore, Option (A) is the correct answer.
Additional information:
It is used for minor aches and mild to moderate pain which is recommended for arthritic conditions. It reduces risk of transient ischemic attacks in men. In organic chemistry, electrophile reaction in a chemical compound, the bond is broken and two new bonds are formed.
Note: In this reaction electron substitution reaction takes place. Here we remember how bond breaks and new bonds formed. The reaction takes place in the presence of a catalyst. Now we try to understand the formation of the product. There are acetic acid and aspirin given to us as products.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




