
The major product obtained in the given reaction is:
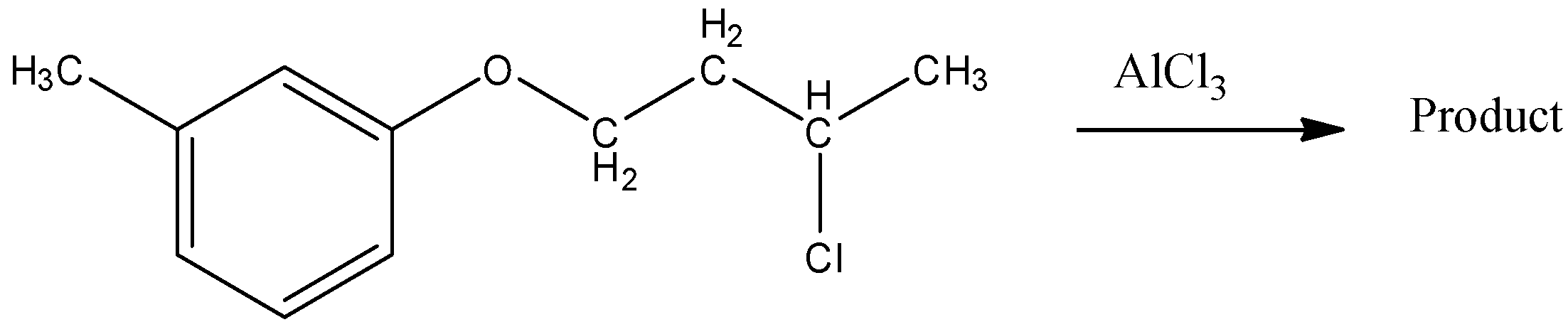
(A)
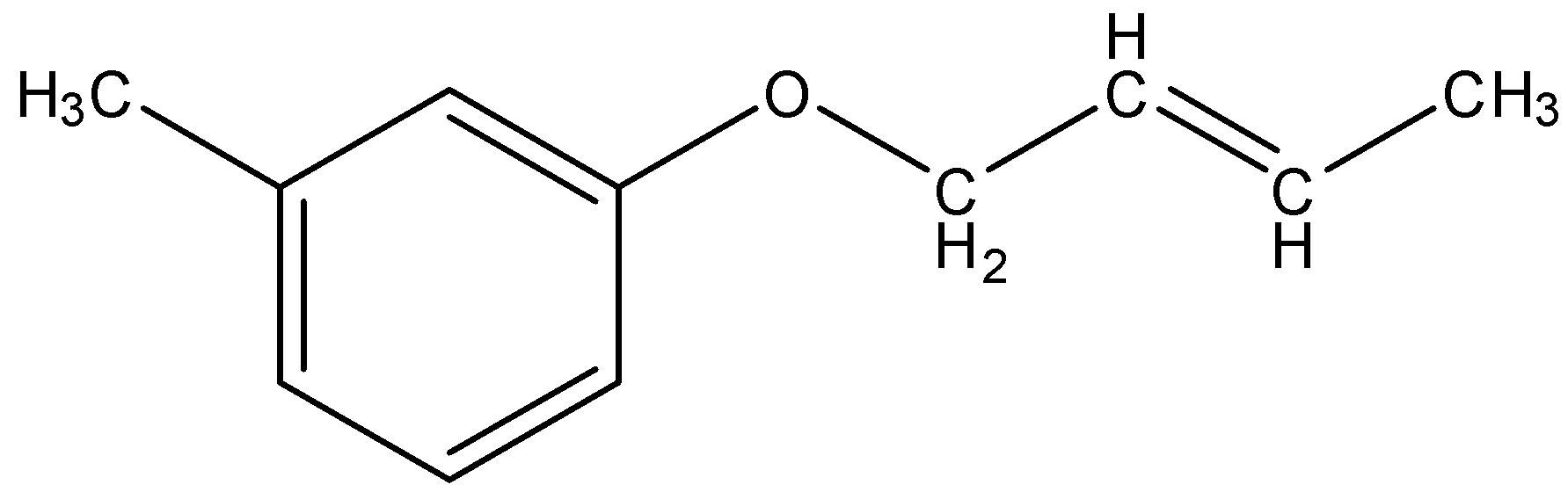
(B)
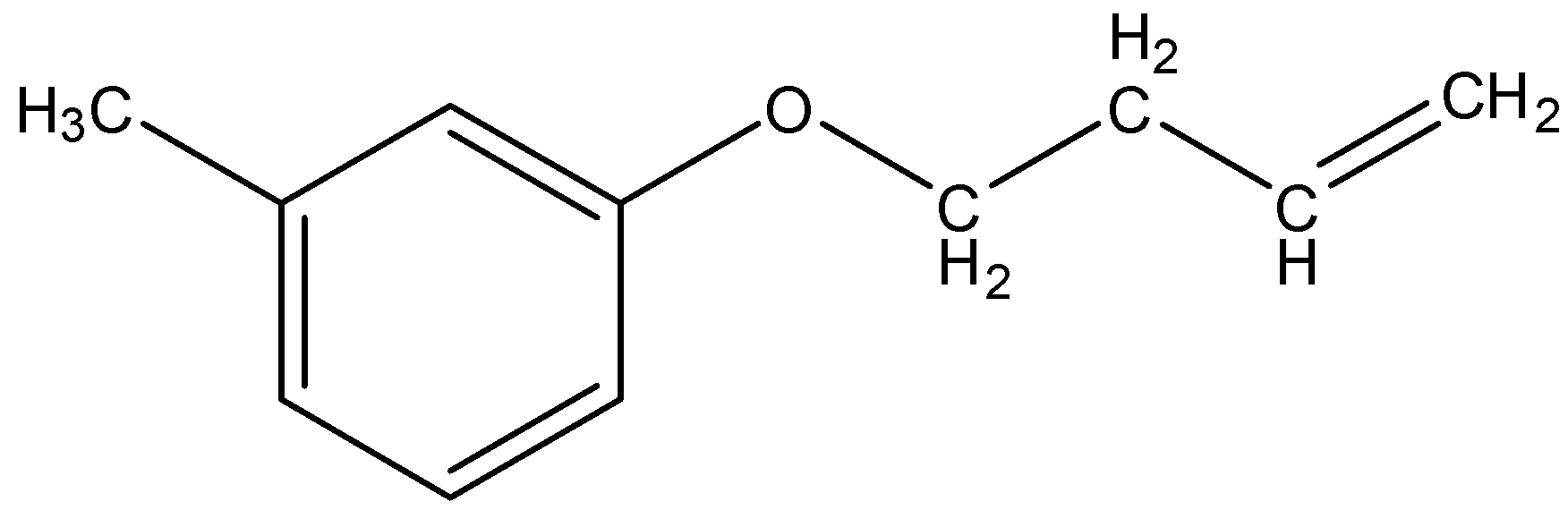
(C)
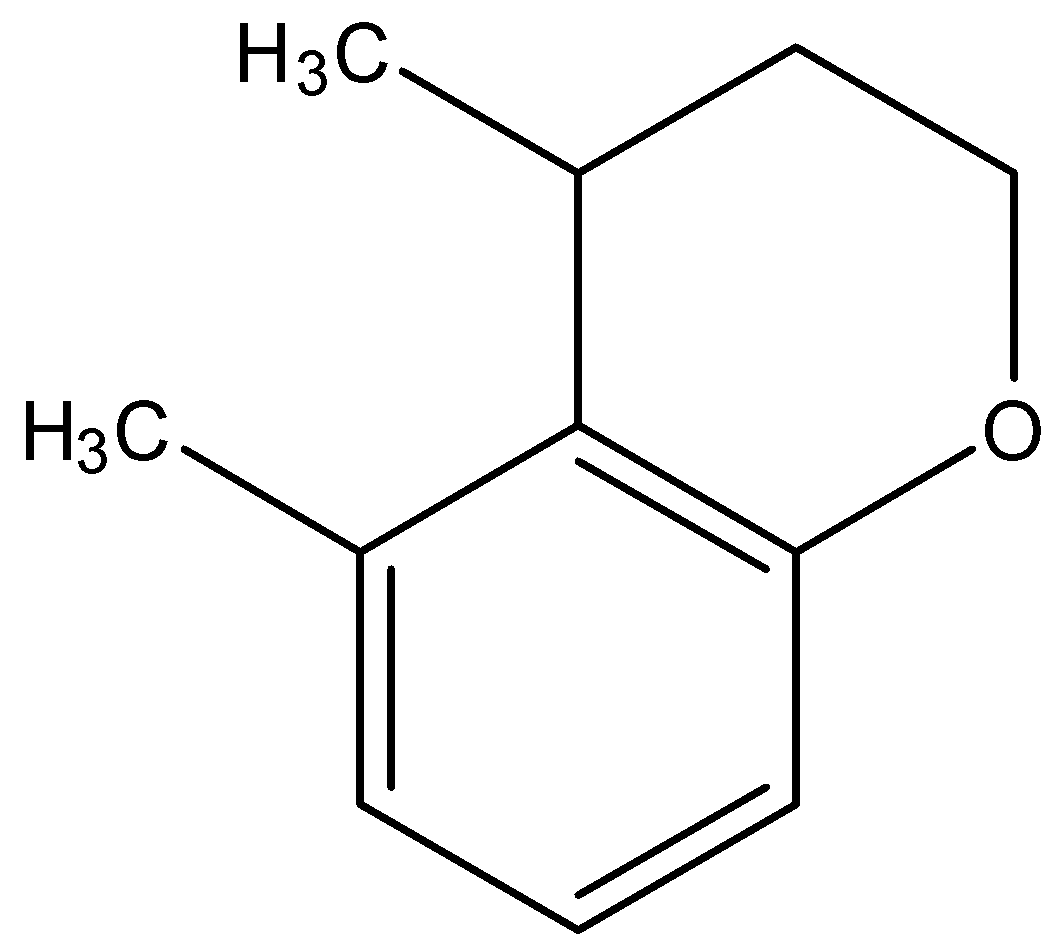
(D)
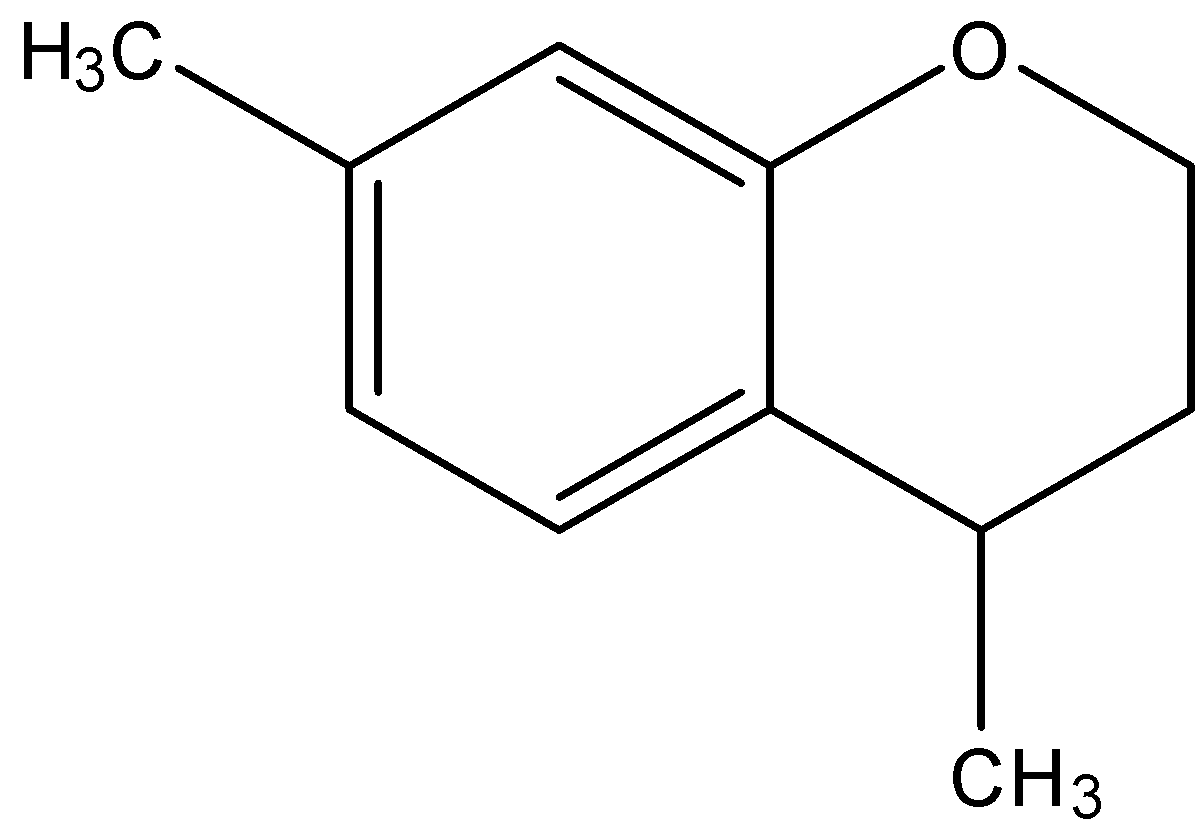





Answer
578.1k+ views
Hint:. Aluminum chloride ($AlC{l_3}$) is a lewis acid because it can accept electron pairs from bases. In presence of aluminum chloride, the compound can give Friedel Crafts reaction if suitable structure is present.
Complete step by step answer:
We can see that an aromatic compound is given in the reaction. It is allowed to react with aluminum chloride.
- We know that $AlC{l_3}$ is a lewis acid. It can give Friedel Crafts reactions.
-Here, the compound has a halogen group on the side chain. This halogen can leave to produce a carbocation which can undergo substitution reaction.
So, the mechanism of the reaction can be given as below.
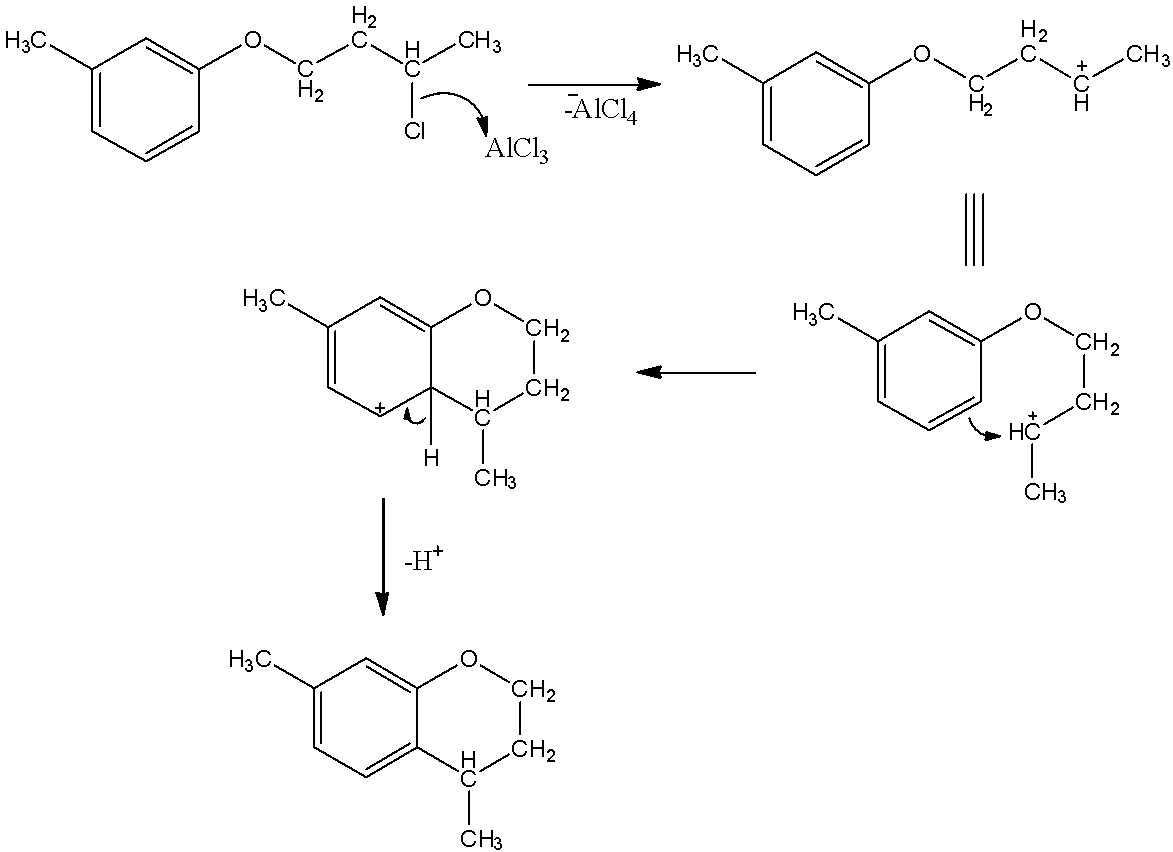
- Here, we can see that in the first step, $AlC{l_3}$ acts as a lewis acid and accepts the electron pair from the chlorine atom. This forms $^ - AlC{l_4}$ . Alongside this, a carbocation is also formed.
- Now, this carbocation is an electrophilic carbon. So, benzene rings can give electrophilic substitution reactions here. So, as a result, the double bond of benzene will attack on the carbocation and a C-C bond will be formed there. Then after losing a proton, the carbon ring becomes aromatic. This, we obtain a compound which has two ring structures. One ring is aromatic and the other is not aromatic.
So, the correct answer is “Option D”.
Note: Note that here the carbocation formed is a secondary carbocation. So, any rearrangement will not occur here because the neighbouring carbons are also secondary carbons. If a tertiary carbon would have been there bonded with the carbocation, then rearrangement would have occurred.
Complete step by step answer:
We can see that an aromatic compound is given in the reaction. It is allowed to react with aluminum chloride.
- We know that $AlC{l_3}$ is a lewis acid. It can give Friedel Crafts reactions.
-Here, the compound has a halogen group on the side chain. This halogen can leave to produce a carbocation which can undergo substitution reaction.
So, the mechanism of the reaction can be given as below.

- Here, we can see that in the first step, $AlC{l_3}$ acts as a lewis acid and accepts the electron pair from the chlorine atom. This forms $^ - AlC{l_4}$ . Alongside this, a carbocation is also formed.
- Now, this carbocation is an electrophilic carbon. So, benzene rings can give electrophilic substitution reactions here. So, as a result, the double bond of benzene will attack on the carbocation and a C-C bond will be formed there. Then after losing a proton, the carbon ring becomes aromatic. This, we obtain a compound which has two ring structures. One ring is aromatic and the other is not aromatic.
So, the correct answer is “Option D”.
Note: Note that here the carbocation formed is a secondary carbocation. So, any rearrangement will not occur here because the neighbouring carbons are also secondary carbons. If a tertiary carbon would have been there bonded with the carbocation, then rearrangement would have occurred.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




