
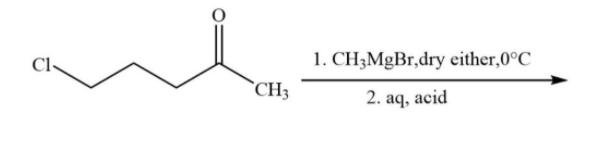
The major product in the following reaction is:
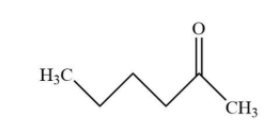
A.
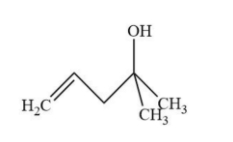
B.
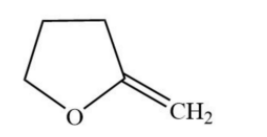
C.
D.
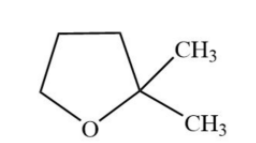





Answer
579.9k+ views
Hint: We know that $C{H_3}MgBr$ is known as Grignard reagent. The more electronegative an element is, the more it attracts the electron density in the bond. In the Grignard reagent, carbon of the alkyl group possesses negative charge and magnesium has positive charge.
Complete answer
Grignard reagents are made by reacting magnesium turnings with alkyl halides in ether solvents to form solutions of alkyl magnesium halide. Iodides, bromides, and chlorides can be used, as can both aryl and alkyl halides, though they cannot contain any functional groups that would react with the Grignard reagent once it is formed.
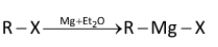
Where, R can be any alkyl/aryl while X is halogen.
Overall, it involves an insertion of magnesium into the new carbon–halogen bond. There is also a change in oxidation state of the magnesium, from Mg (0) to Mg (II). The reaction is therefore known as an oxidative insertion or oxidative addition.
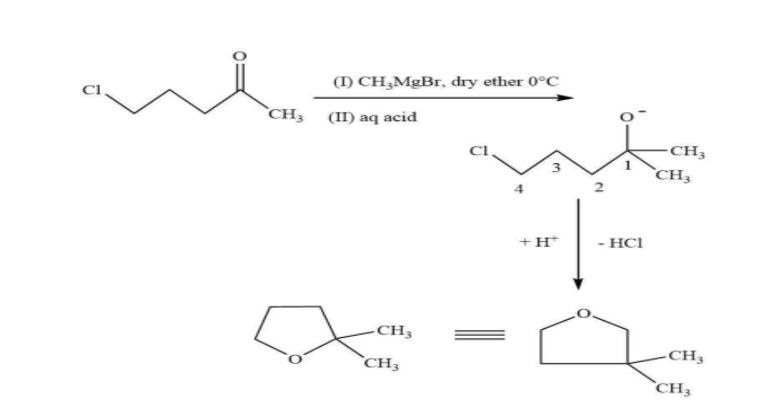
In this reaction, in the first step, the nucleophilic methyl group will attack on the electrophilic end of the carbonyl centre. Due to which a negative charge is generated on oxygen atom then in the second step there is a bimolecular substitution reaction on carbon containing chloride and chloride is removed. And overall, there is a formation of five membered compounds.
Hence, option (D) is the correct option.
Note:
Grignard reagents easily remove acidic protons. These strong nucleophilic reagents like Grignard reagents and organolithiums are strong bases, and may need protection from acidic protons as well as from electrophilic carbonyl groups. Among the most troublesome are the protons of hydroxyl groups.
Complete answer
Grignard reagents are made by reacting magnesium turnings with alkyl halides in ether solvents to form solutions of alkyl magnesium halide. Iodides, bromides, and chlorides can be used, as can both aryl and alkyl halides, though they cannot contain any functional groups that would react with the Grignard reagent once it is formed.

Where, R can be any alkyl/aryl while X is halogen.
Overall, it involves an insertion of magnesium into the new carbon–halogen bond. There is also a change in oxidation state of the magnesium, from Mg (0) to Mg (II). The reaction is therefore known as an oxidative insertion or oxidative addition.

In this reaction, in the first step, the nucleophilic methyl group will attack on the electrophilic end of the carbonyl centre. Due to which a negative charge is generated on oxygen atom then in the second step there is a bimolecular substitution reaction on carbon containing chloride and chloride is removed. And overall, there is a formation of five membered compounds.
Hence, option (D) is the correct option.
Note:
Grignard reagents easily remove acidic protons. These strong nucleophilic reagents like Grignard reagents and organolithiums are strong bases, and may need protection from acidic protons as well as from electrophilic carbonyl groups. Among the most troublesome are the protons of hydroxyl groups.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




