
What would be the major product for the given electrolysis reaction.
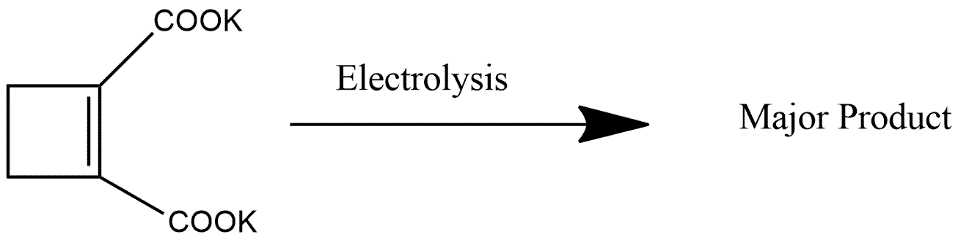
(1)
(2)
(3)
(4) None




Answer
567.6k+ views
Hint: Butene generally contains a chain of 4 carbon atoms having an alkene group when it undergoes an electrolysis process then this type of reactions are known as Kolbe reactions which is also called Kolbe Schmitt Reaction. It is generally an additional reaction.
Complete Step by step solution: Kolbe electrolysis is generally an electrochemical decarboxylation of the salts of carboxylic acid which produces radicals. This reaction is also called as decarboxylative dimerization because it undergoes radical reaction mechanisms. This reaction is used in the synthesis of symmetrical dimers. In the given reaction COOK are given as carboxylic acid salts which undergo through the process of dimerization and it generally follows two step mechanism which is described as:
First of all we know that
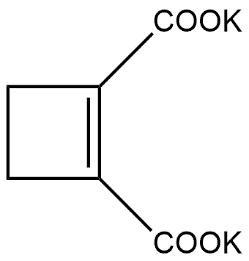
Can also be written as
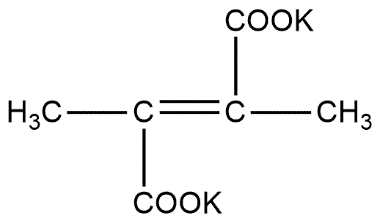
In first step in undergoes through the process of electrolysis and forms radicals as shown below:
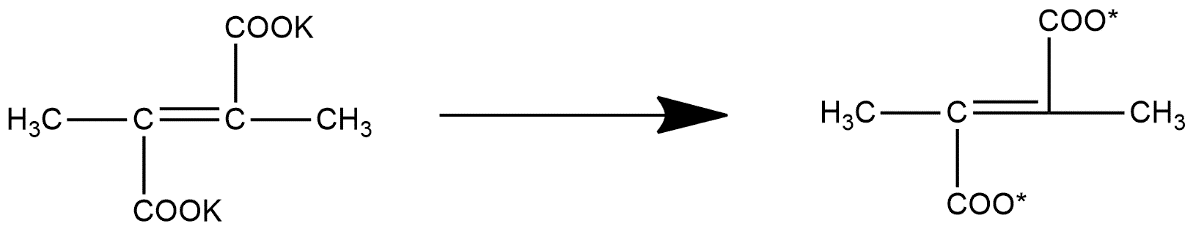
But these are not so stable so it readily losses carbon dioxide atoms and from alkyne group which can be represented as:
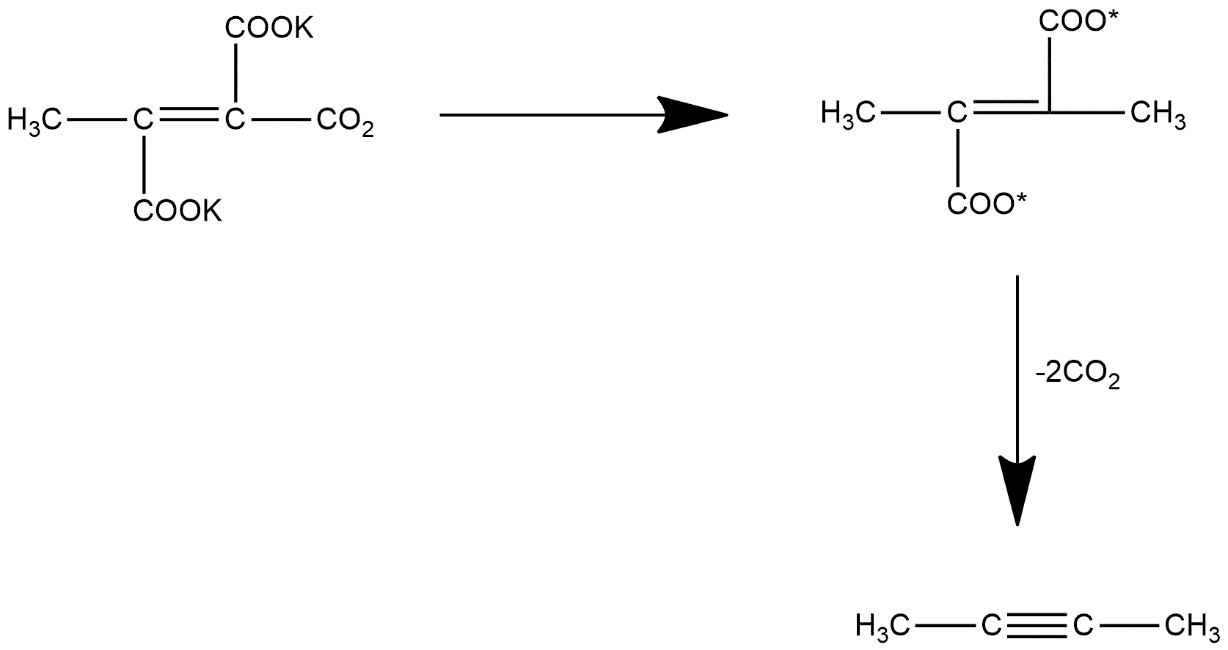
Product formed is 2-butyne which can also be represented as:
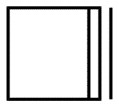
Hence we can say that option 1 is the correct answer.
Note: Radical substitution reaction is a type of reaction which occurs by a free radical mechanism which results in the substitution of one or more of the atoms or groups present in the substrate by different atoms or groups. In the initiation step of radical chain reaction firstly free radical is produced and in terminating step two radicals react together in some way so that the chain can no longer be propagated.
Complete Step by step solution: Kolbe electrolysis is generally an electrochemical decarboxylation of the salts of carboxylic acid which produces radicals. This reaction is also called as decarboxylative dimerization because it undergoes radical reaction mechanisms. This reaction is used in the synthesis of symmetrical dimers. In the given reaction COOK are given as carboxylic acid salts which undergo through the process of dimerization and it generally follows two step mechanism which is described as:
First of all we know that

Can also be written as

In first step in undergoes through the process of electrolysis and forms radicals as shown below:

But these are not so stable so it readily losses carbon dioxide atoms and from alkyne group which can be represented as:

Product formed is 2-butyne which can also be represented as:

Hence we can say that option 1 is the correct answer.
Note: Radical substitution reaction is a type of reaction which occurs by a free radical mechanism which results in the substitution of one or more of the atoms or groups present in the substrate by different atoms or groups. In the initiation step of radical chain reaction firstly free radical is produced and in terminating step two radicals react together in some way so that the chain can no longer be propagated.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




