
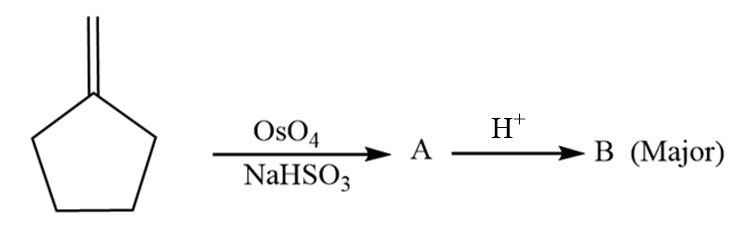
The major product ‘B’ in the given reaction sequence is:
A.
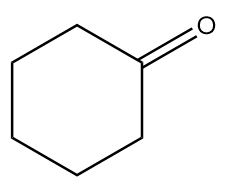
B.
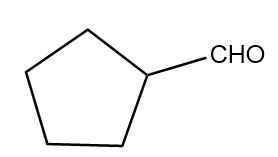
C.
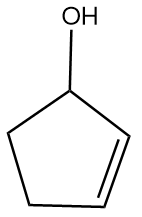
D.





Answer
506.7k+ views
Hint: Osmium tetroxide is a volatile liquid with chemical formula $ Os{O_4} $ and reacts with alkene to form cis-diols. Osmium tetroxide is at advantage over potassium permanganate because it is much more compatible with other functional groups and is performed in mild conditions.
Complete answer:
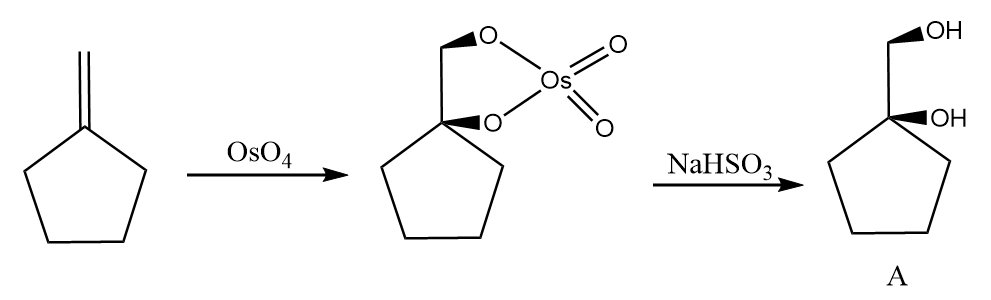
The reaction of alkene with $ Os{O_4} $ work through a concerted process where two oxygen atoms from the osmium tetroxide interact with the double bond of the alkene results in formation of a five membered ring which is known as osmate ester with syn stereochemistry. The osmate ester is broken into diol in the presence of reducing agents such as sodium bisulphite $ (NaHS{O_3}) $ . The given reaction proceeds as follows:
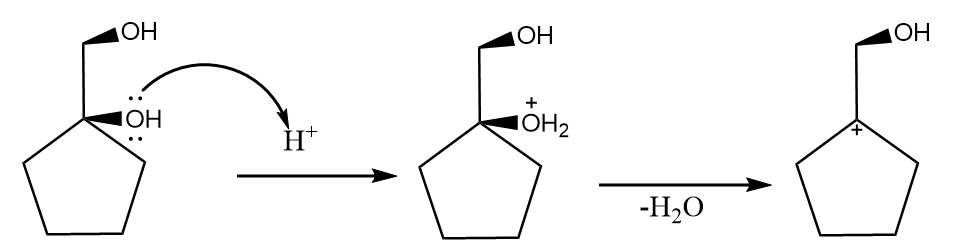
Now, when the product A i.e., diol is further reacted with hydrogen ion, then the dehydration reaction takes place along with the formation of a carbocation. The reaction proceeds as follows:
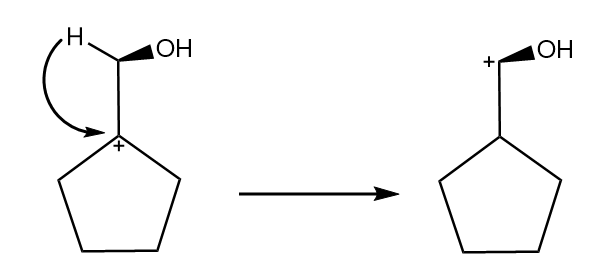
Hydride shift will take place and a new carbocation will be formed as follows:
The oxygen atom behaves as a nucleophile and donates its lone pair of electrons to the carbocation by forming a double bond and removal of hydrogen ion takes place. The reaction proceeds as follows:
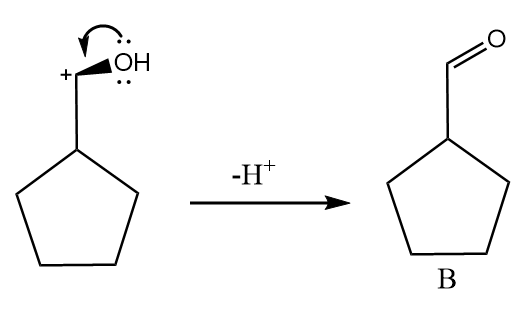
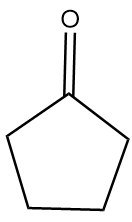
Hence, the major product B formed in the given reaction sequence is cyclopentane carbaldehyde and its structurally represented as follows:
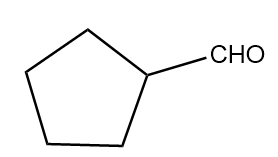
Thus, option (B) is the correct answer.
Note:
It is important to note that as alkene is planar in structure, the attack of osmium tetroxide can either be above the plane or below the plane of alkene. Also, while hydrogen ions attack on diol, there is another possible carbocation which is a primary carbocation and hence, unstable. Therefore, the product formed by primary carbocation is considered as a minor product.
Complete answer:
The reaction of alkene with $ Os{O_4} $ work through a concerted process where two oxygen atoms from the osmium tetroxide interact with the double bond of the alkene results in formation of a five membered ring which is known as osmate ester with syn stereochemistry. The osmate ester is broken into diol in the presence of reducing agents such as sodium bisulphite $ (NaHS{O_3}) $ . The given reaction proceeds as follows:

Now, when the product A i.e., diol is further reacted with hydrogen ion, then the dehydration reaction takes place along with the formation of a carbocation. The reaction proceeds as follows:

Hydride shift will take place and a new carbocation will be formed as follows:

The oxygen atom behaves as a nucleophile and donates its lone pair of electrons to the carbocation by forming a double bond and removal of hydrogen ion takes place. The reaction proceeds as follows:

Hence, the major product B formed in the given reaction sequence is cyclopentane carbaldehyde and its structurally represented as follows:

Thus, option (B) is the correct answer.
Note:
It is important to note that as alkene is planar in structure, the attack of osmium tetroxide can either be above the plane or below the plane of alkene. Also, while hydrogen ions attack on diol, there is another possible carbocation which is a primary carbocation and hence, unstable. Therefore, the product formed by primary carbocation is considered as a minor product.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




