
The major product ( $ 70\% $ to $ 80\% $ ) of the reaction between m-dinitrobenzene with $ N{H_4}HS $ is:
(A)
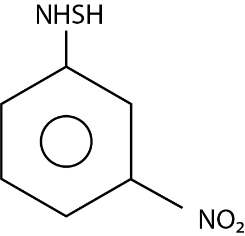
(B)
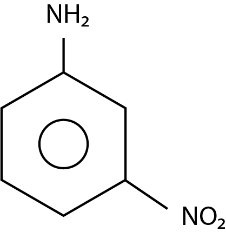
(C)
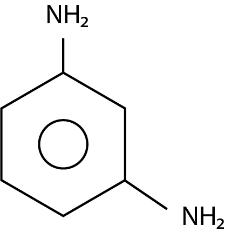
(D)
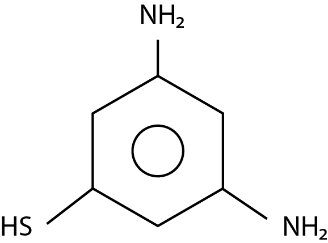




Answer
547.5k+ views
Hint: $ N{H_4}HS $ (ammonium bisulfide), helps in the selective reduction of nitro group to amine group. Selective reduction refers to the reduction of any one of the groups where two similar groups are present. In m-dinitrobenzene, two nitro groups are present. Out of two nitro groups, one will be reduced to $ - N{H_2} $ group. No further effect of reagent will be there on the other nitro group.
Complete step by step solution:
The given reactant is m-dinitrobenzene.
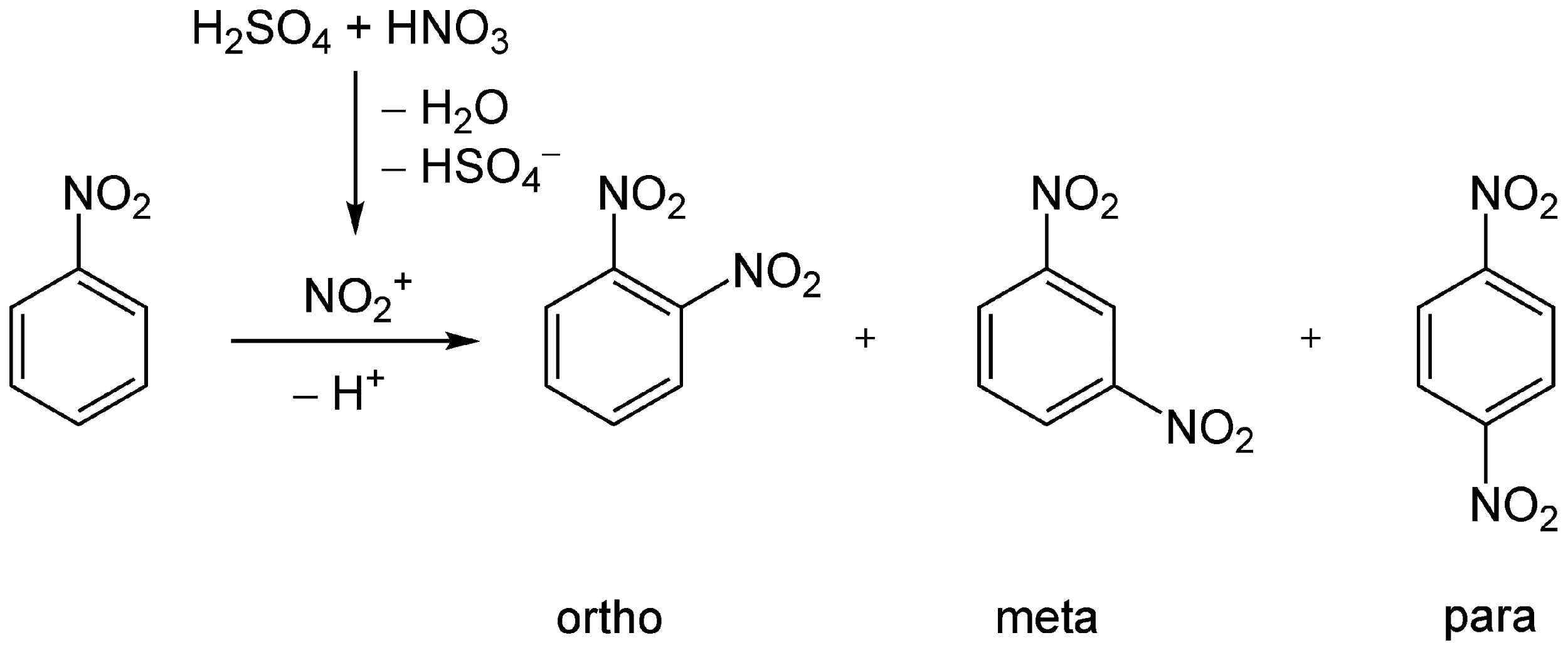
Reduction of dinitrobenzene with ammonium sulphide reduces only one nitro group. This reduction is called zenin reduction.
The desired reaction is given below:
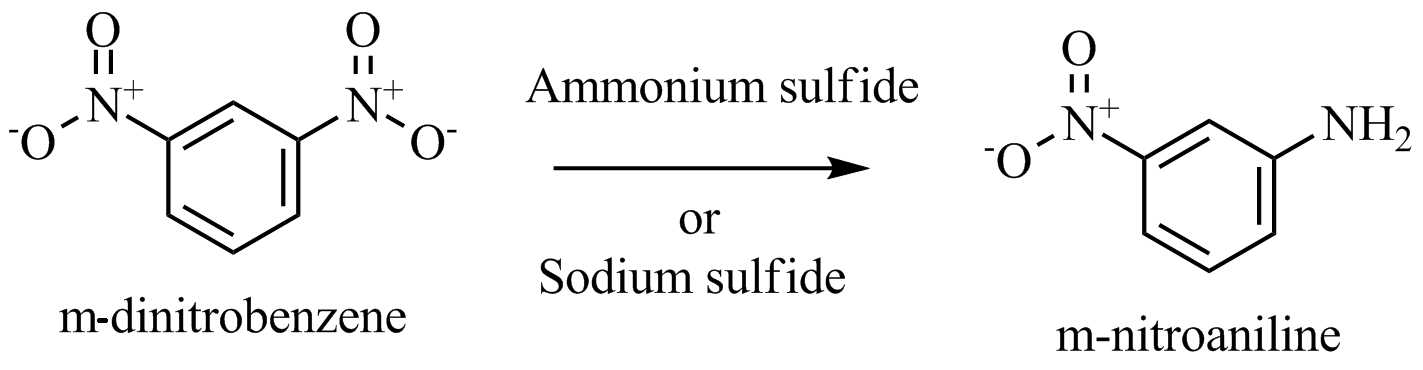
Here, we can see that one nitro group is reduced to an amino group while the other one left undisturbed. As the compound is symmetric, we can reduce any nitro group according to our choice. Because both are the same with respect to one another.
Moreover, if we want to reduce the leftover nitro group, it can also be done with the help of $ Sn/HCl $ . Further reduction with $ Sn/HCl $ given m-phenylenediamine.
So, the major product ( $ 70\% $ to $ 80\% $ ) of the reaction between m-dinitrobenzene with $ N{H_4}HS $ is
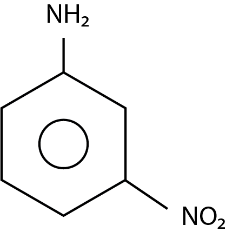
Hence, the correct option is (C).
Note:
Same reduction can also be carried out with the help of sodium sulfide in aqueous solution. Further reduction with iron and hydrochloric acid gives m-phenylenediamine. m-dinitrobenzene is an organic compound which has three isomers. It is a yellow solid that is soluble in organic solvents.
Complete step by step solution:
The given reactant is m-dinitrobenzene.

Reduction of dinitrobenzene with ammonium sulphide reduces only one nitro group. This reduction is called zenin reduction.
The desired reaction is given below:

Here, we can see that one nitro group is reduced to an amino group while the other one left undisturbed. As the compound is symmetric, we can reduce any nitro group according to our choice. Because both are the same with respect to one another.
Moreover, if we want to reduce the leftover nitro group, it can also be done with the help of $ Sn/HCl $ . Further reduction with $ Sn/HCl $ given m-phenylenediamine.
So, the major product ( $ 70\% $ to $ 80\% $ ) of the reaction between m-dinitrobenzene with $ N{H_4}HS $ is

Hence, the correct option is (C).
Note:
Same reduction can also be carried out with the help of sodium sulfide in aqueous solution. Further reduction with iron and hydrochloric acid gives m-phenylenediamine. m-dinitrobenzene is an organic compound which has three isomers. It is a yellow solid that is soluble in organic solvents.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




