
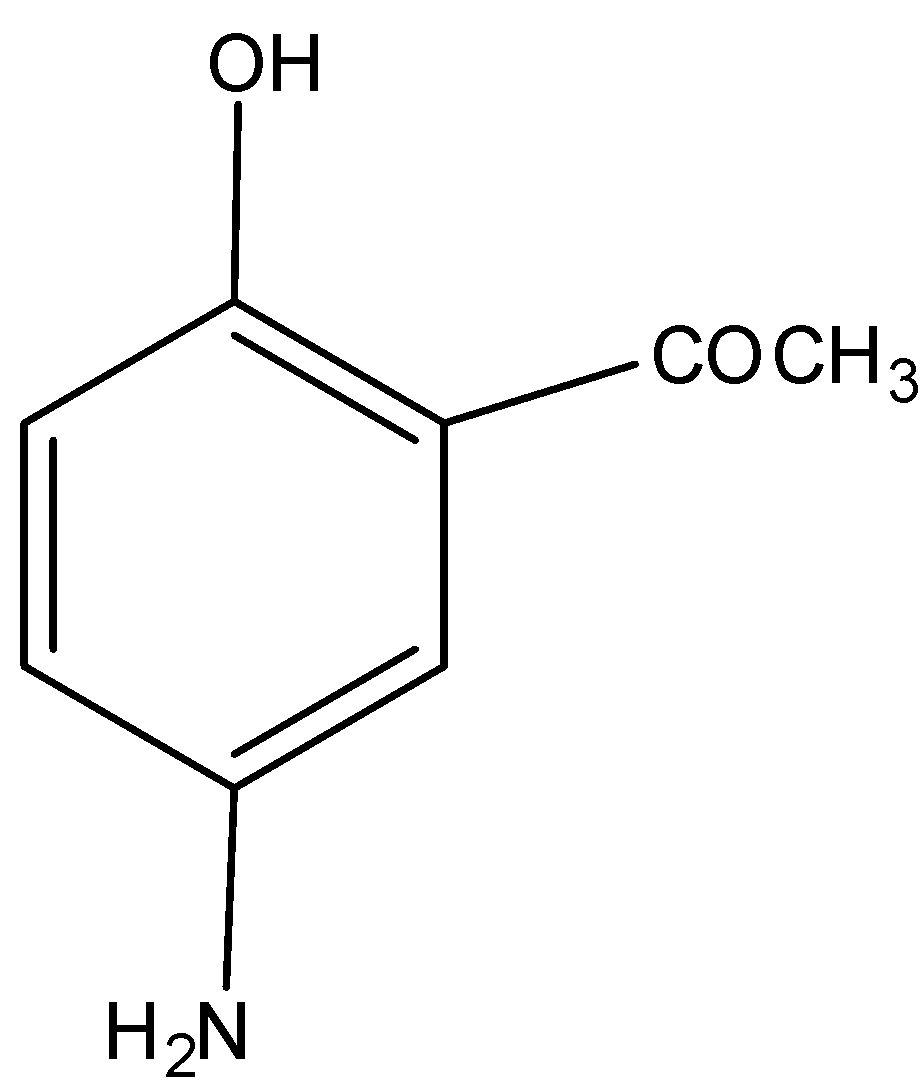
The main product formed in the following reaction is.
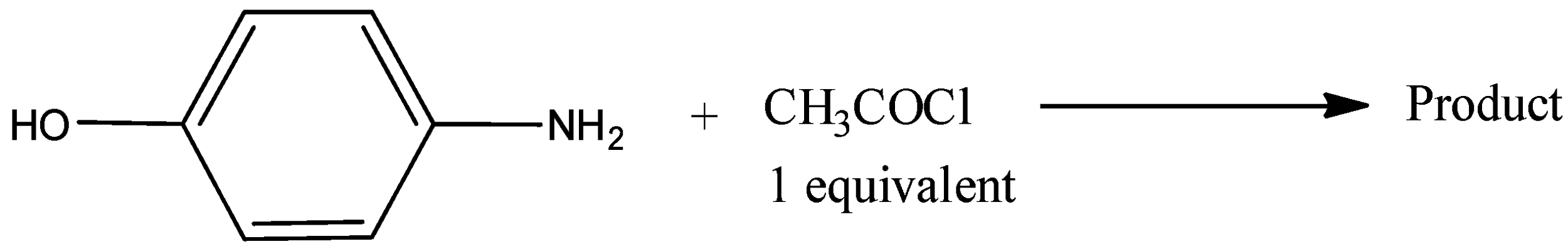
A)
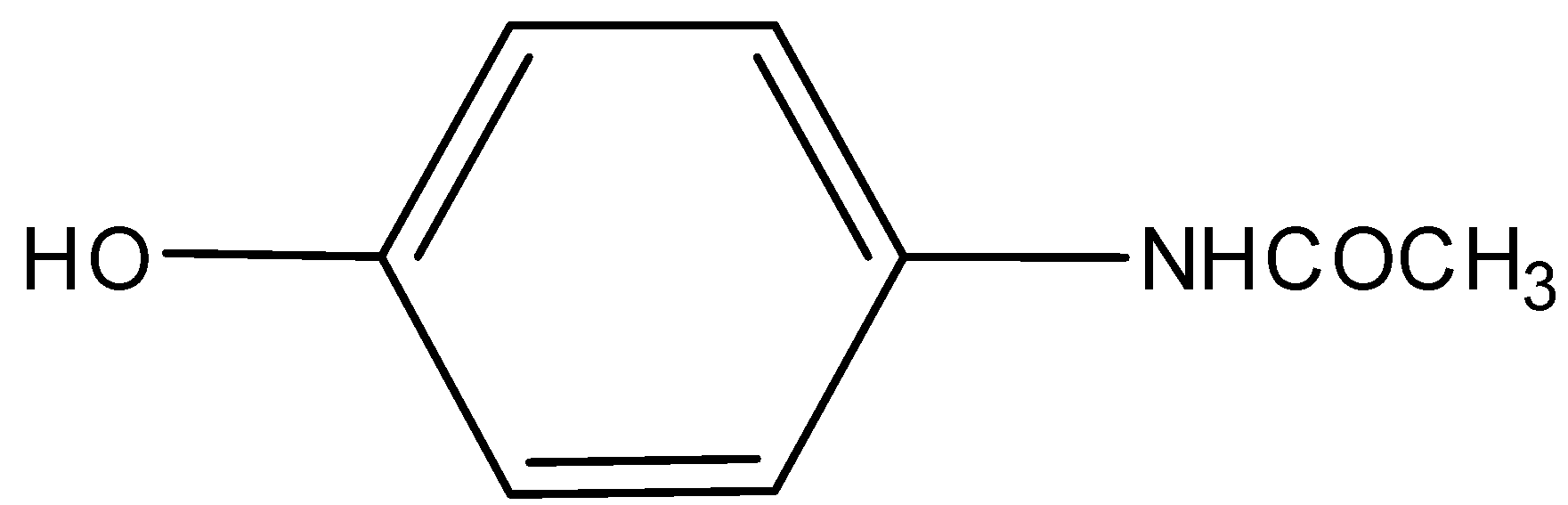
B)
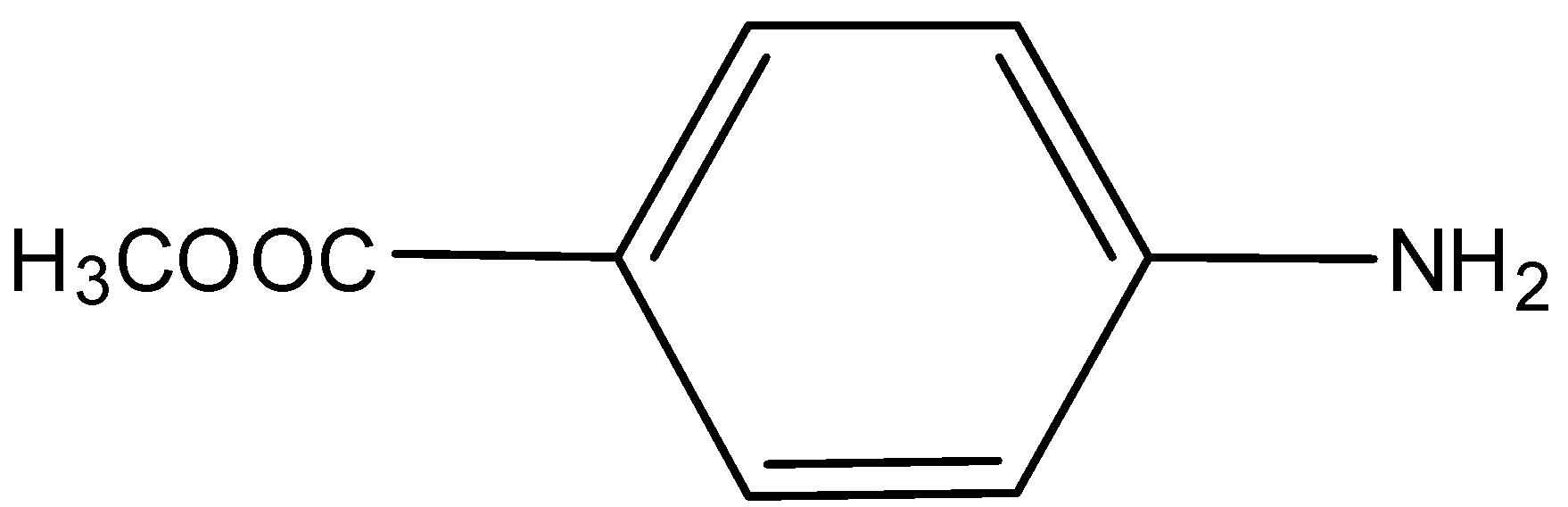
C)
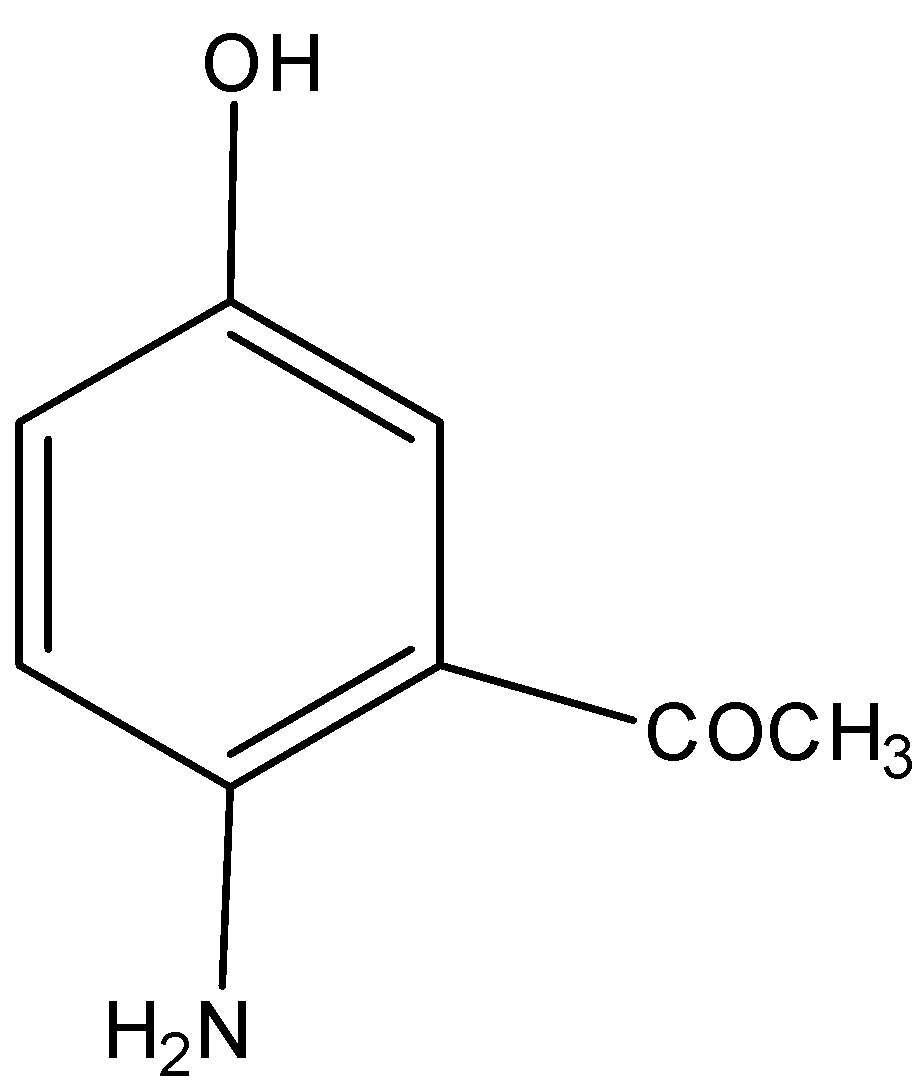
D)
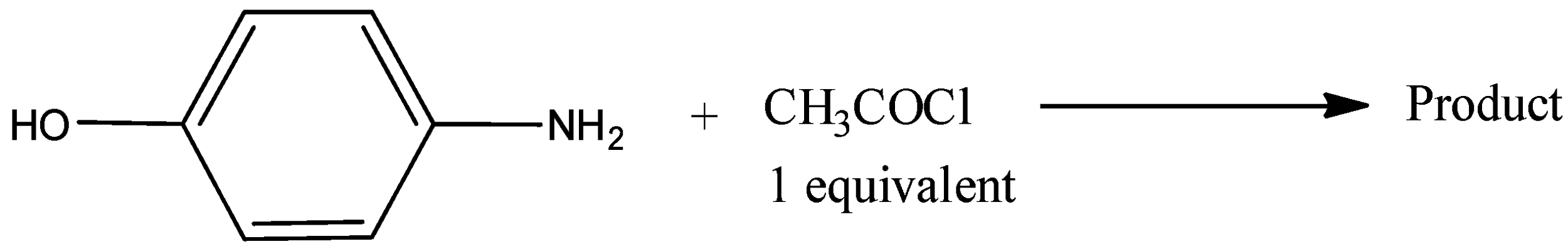




Answer
550.8k+ views
Hint: We know that 4-Aminophenol (or para-aminophenol or p-aminophenol) is the natural compound with the recipe ${H_2}N{C_6}{H_4}OH$. Normally accessible as a white powder it was ordinarily utilized as a designer for highly contrasting film, advertised under the name Rodinal.
Complete step by step answer:
We all know that hydroxyl group is less reactive than the amine group thus amine group will attack acetyl chloride group and give acetaminophen commonly called paracetamol.
The reaction is,
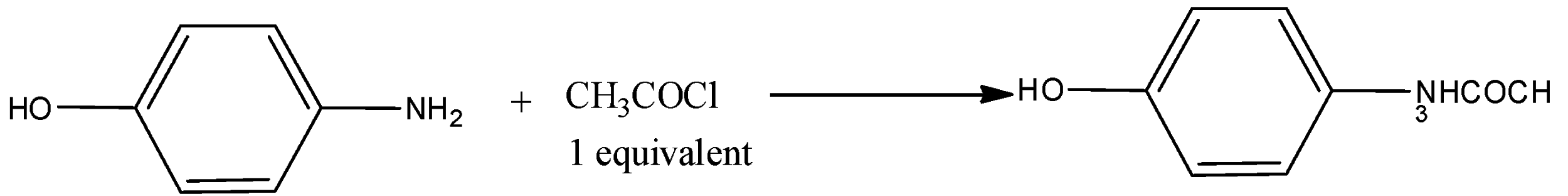 Thus option A is correct.
Thus option A is correct.
As we know that the paracetamol is a drug used to treat torment and fever. It is regularly utilized for mellow to direct torment relief. Evidence is blended for its utilization to assuage fever in children. It is frequently sold in mix with different meds, for example, in numerous cold medications.
Paracetamol is likewise utilized for serious torment, for example, malignant growth agony and torment after a medical procedure, in mix with narcotic torment medication. It is ordinarily used either by mouth or rectally, and yet is open by implantation into a vein. Impacts last some place in the scope of two and four hours.
So, the correct answer is Option A.
Note: We know that paracetamol is utilized for decreasing fever in individuals of all ages. The World Health Organization prescribes that paracetamol be utilized to treat fever in youngsters just if their temperature is higher than \[38.5^\circ C\left( {101.3^\circ F} \right)\]. The viability of paracetamol without help from anyone else in kids with fevers has been questioned and a meta-investigation demonstrated that it is less powerful than ibuprofen. Paracetamol doesn't have critical calming impacts.
Complete step by step answer:
We all know that hydroxyl group is less reactive than the amine group thus amine group will attack acetyl chloride group and give acetaminophen commonly called paracetamol.
The reaction is,
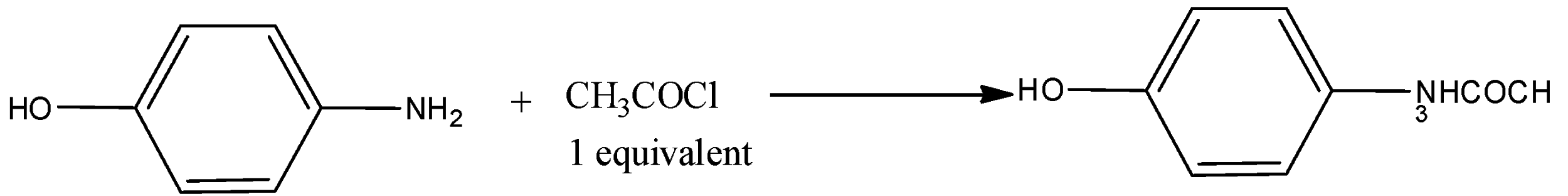
As we know that the paracetamol is a drug used to treat torment and fever. It is regularly utilized for mellow to direct torment relief. Evidence is blended for its utilization to assuage fever in children. It is frequently sold in mix with different meds, for example, in numerous cold medications.
Paracetamol is likewise utilized for serious torment, for example, malignant growth agony and torment after a medical procedure, in mix with narcotic torment medication. It is ordinarily used either by mouth or rectally, and yet is open by implantation into a vein. Impacts last some place in the scope of two and four hours.
So, the correct answer is Option A.
Note: We know that paracetamol is utilized for decreasing fever in individuals of all ages. The World Health Organization prescribes that paracetamol be utilized to treat fever in youngsters just if their temperature is higher than \[38.5^\circ C\left( {101.3^\circ F} \right)\]. The viability of paracetamol without help from anyone else in kids with fevers has been questioned and a meta-investigation demonstrated that it is less powerful than ibuprofen. Paracetamol doesn't have critical calming impacts.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




