
The long form of periodic table consists of:
A) Seven periods and eight groups
B) Seven periods and eighteen groups
C) Eight periods and eighteen groups
D) Eighteen periods and eight groups.
Answer
570.9k+ views
Hint:The long form of periodic table or modern periodic table is a tabular arrangement of all the chemical elements that have been discovered or invented so far. This periodic table is based on the modern periodic law.
Complete step-by-step answer:Let us understand the modern periodic table. The basis of this table is atomic number and hence the modern periodic table or the long form of periodic table states that the physical and chemical properties of elements are periodic functions of their atomic number.
The modern periodic table consists of elements with their atomic number shown on top of their symbol and their atomic mass shown below their symbol. An element represented in the periodic table looks like the following figure.
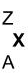
Here, X is the symbol of an element.
Z is the atomic number of the element.
A is the atomic mass of the element.
These elements are represented in an ascending order of their atomic number from left to right. Currently there are one hundred and eight elements in the periodic table.
There are seven periods and eighteen groups in a periodic table.
Therefore the correct answer is option D.
Note:It is to be noted that a period consists of elements in the horizontal line and a group consists of elements on the vertical line. It should also be noted that the elements belonging to the same group show some similarities in their chemical and physical properties.
Complete step-by-step answer:Let us understand the modern periodic table. The basis of this table is atomic number and hence the modern periodic table or the long form of periodic table states that the physical and chemical properties of elements are periodic functions of their atomic number.
The modern periodic table consists of elements with their atomic number shown on top of their symbol and their atomic mass shown below their symbol. An element represented in the periodic table looks like the following figure.
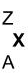
Here, X is the symbol of an element.
Z is the atomic number of the element.
A is the atomic mass of the element.
These elements are represented in an ascending order of their atomic number from left to right. Currently there are one hundred and eight elements in the periodic table.
There are seven periods and eighteen groups in a periodic table.
Therefore the correct answer is option D.
Note:It is to be noted that a period consists of elements in the horizontal line and a group consists of elements on the vertical line. It should also be noted that the elements belonging to the same group show some similarities in their chemical and physical properties.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




