
The List-I below gives some quantities involved in a process and List-II gives some possible values of these quantities.
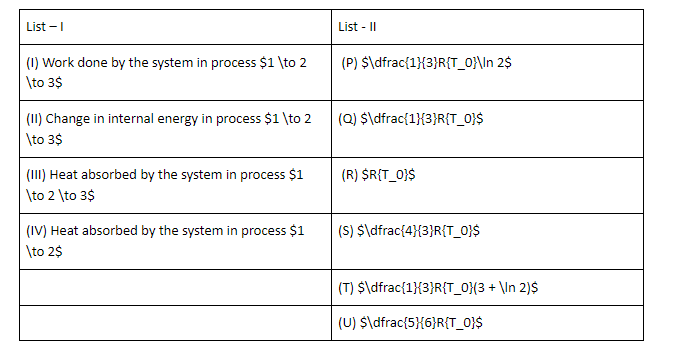
If the process carried out on one mole of monatomic ideal gas is as shown in figure in the PV-diagram with ${P_0}{V_0} = \dfrac{1}{3}R{T_0}$ the correct match is,
A. I → S, II → R, III → Q, IV → T
B. I → Q, II → R, III → P, IV → U
C. I → Q, II → S, III → R, IV → U
D. I → Q, II → R, III → S, IV → U

Answer
566.7k+ views
Hint: Ideal gas is a perfect gas in which there will be no interaction between the constituent molecules. One molecule will not be affected by the other molecule. These molecules occupy a negligible amount of space and they always obey gas laws. We solve this problem using the gas laws.
Formula used:
$\eqalign{
& \Delta Q = \Delta U + \Delta W \cr
& \Delta U = n{C_V}\Delta T \cr
& \Delta W = P\Delta V \cr
& {C_V} = \dfrac{f}{2}R \cr
& \Delta PV = nR\Delta T \cr} $
Complete step by step answer:
By referring the diagram below we will try to answer the questions asked
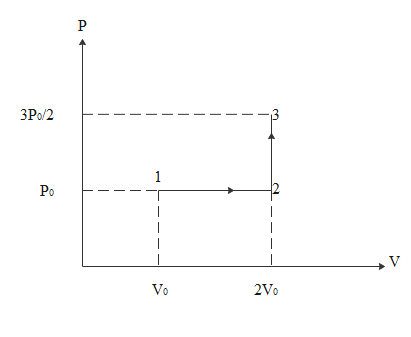
Work done in the process 1-2-3 will be equal to work done in 1-2 only. This is because the formula for work done is $\Delta W = P\Delta V$
In the process 2-3 changes in volume is zero. so work done will be zero
For the process 1-2 work done will be
$\Delta W = P\Delta V$
$\eqalign{
& \Rightarrow \Delta W = P({V_2} - {V_1}) \cr
& \Rightarrow \Delta W = {P_0}(2{V_0} - {V_0}) \cr
& \Rightarrow \Delta W = {P_0}{V_0} \cr
& \Rightarrow {P_0}{V_0} = \dfrac{1}{3}R{T_0} \cr
& \therefore \Delta W = \dfrac{1}{3}R{T_0} \cr} $
Change in internal energy in process $1 \to 2 \to 3$ is $\Delta U = n{C_V}\Delta T$
The degrees of freedom(f) for monatomic gas is 3. Number of moles(n) is one.
For ideal gas $\Delta PV = nR\Delta T$
$\Delta U = n{C_V}\Delta T$
$\eqalign{
& \Rightarrow \Delta U = n{C_V}\Delta T \cr
& \Rightarrow \Delta U = n\left( {\dfrac{f}{2}R} \right)\Delta T \cr
& \Rightarrow f = 3 \cr
& \Rightarrow \Delta U = n\left( {\dfrac{3}{2}R} \right)\Delta T \cr
& \Rightarrow \Delta U = \dfrac{3}{2}nR\Delta T \cr
& \Rightarrow \Delta U = \dfrac{3}{2}(\Delta PV) \cr
& \Rightarrow \Delta U = \dfrac{3}{2}({P_3}{V_3} - {P_1}{V_1}) \cr
& \Rightarrow \Delta U = \dfrac{3}{2}(\dfrac{{3{P_0}}}{2}\left( {2{V_0}} \right) - {P_0}{V_0}) \cr
& \Rightarrow \Delta U = 3{P_0}{V_0} \cr
& \Rightarrow {P_0}{V_0} = \dfrac{1}{3}R{T_0} \cr
& \therefore \Delta U = R{T_0} \cr} $
Heat absorbed by the system in process $1 \to 2 \to 3$ will be found out by using first law of thermodynamics $\Delta Q = \Delta U + \Delta W$
It states that the amount of heat given is used to do work on the system and to raise the internal energy of the system.
$\Delta Q = \Delta U + \Delta W$
$ \Rightarrow \Delta U = R{T_0}$
$ \Rightarrow \Delta W = \dfrac{1}{3}R{T_0}$
$\eqalign{
& \Rightarrow \Delta Q = R{T_0} + \dfrac{1}{3}R{T_0} \cr
& \therefore \Delta Q = \dfrac{4}{3}R{T_0} \cr} $
Heat absorbed by the system in process $1 \to 2$ will be found from same equation $\Delta Q = \Delta U + \Delta W$
$\Delta Q = \Delta U + \Delta W$
$\eqalign{
& \Rightarrow \Delta U = \dfrac{3}{2}(\Delta PV) \cr
& \Rightarrow \Delta Q = \dfrac{3}{2}(\Delta PV) + \Delta W \cr
& \Rightarrow \Delta Q = \dfrac{3}{2}({P_0}2{V_0} - {P_0}{V_0}) + {P_0}{V_0} \cr
& \Rightarrow \Delta Q = \dfrac{5}{2}{P_0}{V_0} \cr
& \Rightarrow {P_0}{V_0} = \dfrac{1}{3}R{T_0} \cr
& \Rightarrow \Delta Q = \dfrac{5}{2}\dfrac{{R{T_0}}}{3} \cr
& \therefore \Delta Q = \dfrac{{5R{T_0}}}{6} \cr} $
Hence I → Q, II → R, III → S, IV → U is correct
So option D is correct.
Note:
All the ideal gas laws we apply are valid only for the ideal gases. At low temperatures and high pressures there will be interactions between the molecules of the gases. Hence they deviate from the ideal gas behaviour. Usually gases behave as ideal gases at higher temperatures and lower pressures.
Formula used:
$\eqalign{
& \Delta Q = \Delta U + \Delta W \cr
& \Delta U = n{C_V}\Delta T \cr
& \Delta W = P\Delta V \cr
& {C_V} = \dfrac{f}{2}R \cr
& \Delta PV = nR\Delta T \cr} $
Complete step by step answer:
By referring the diagram below we will try to answer the questions asked

Work done in the process 1-2-3 will be equal to work done in 1-2 only. This is because the formula for work done is $\Delta W = P\Delta V$
In the process 2-3 changes in volume is zero. so work done will be zero
For the process 1-2 work done will be
$\Delta W = P\Delta V$
$\eqalign{
& \Rightarrow \Delta W = P({V_2} - {V_1}) \cr
& \Rightarrow \Delta W = {P_0}(2{V_0} - {V_0}) \cr
& \Rightarrow \Delta W = {P_0}{V_0} \cr
& \Rightarrow {P_0}{V_0} = \dfrac{1}{3}R{T_0} \cr
& \therefore \Delta W = \dfrac{1}{3}R{T_0} \cr} $
Change in internal energy in process $1 \to 2 \to 3$ is $\Delta U = n{C_V}\Delta T$
The degrees of freedom(f) for monatomic gas is 3. Number of moles(n) is one.
For ideal gas $\Delta PV = nR\Delta T$
$\Delta U = n{C_V}\Delta T$
$\eqalign{
& \Rightarrow \Delta U = n{C_V}\Delta T \cr
& \Rightarrow \Delta U = n\left( {\dfrac{f}{2}R} \right)\Delta T \cr
& \Rightarrow f = 3 \cr
& \Rightarrow \Delta U = n\left( {\dfrac{3}{2}R} \right)\Delta T \cr
& \Rightarrow \Delta U = \dfrac{3}{2}nR\Delta T \cr
& \Rightarrow \Delta U = \dfrac{3}{2}(\Delta PV) \cr
& \Rightarrow \Delta U = \dfrac{3}{2}({P_3}{V_3} - {P_1}{V_1}) \cr
& \Rightarrow \Delta U = \dfrac{3}{2}(\dfrac{{3{P_0}}}{2}\left( {2{V_0}} \right) - {P_0}{V_0}) \cr
& \Rightarrow \Delta U = 3{P_0}{V_0} \cr
& \Rightarrow {P_0}{V_0} = \dfrac{1}{3}R{T_0} \cr
& \therefore \Delta U = R{T_0} \cr} $
Heat absorbed by the system in process $1 \to 2 \to 3$ will be found out by using first law of thermodynamics $\Delta Q = \Delta U + \Delta W$
It states that the amount of heat given is used to do work on the system and to raise the internal energy of the system.
$\Delta Q = \Delta U + \Delta W$
$ \Rightarrow \Delta U = R{T_0}$
$ \Rightarrow \Delta W = \dfrac{1}{3}R{T_0}$
$\eqalign{
& \Rightarrow \Delta Q = R{T_0} + \dfrac{1}{3}R{T_0} \cr
& \therefore \Delta Q = \dfrac{4}{3}R{T_0} \cr} $
Heat absorbed by the system in process $1 \to 2$ will be found from same equation $\Delta Q = \Delta U + \Delta W$
$\Delta Q = \Delta U + \Delta W$
$\eqalign{
& \Rightarrow \Delta U = \dfrac{3}{2}(\Delta PV) \cr
& \Rightarrow \Delta Q = \dfrac{3}{2}(\Delta PV) + \Delta W \cr
& \Rightarrow \Delta Q = \dfrac{3}{2}({P_0}2{V_0} - {P_0}{V_0}) + {P_0}{V_0} \cr
& \Rightarrow \Delta Q = \dfrac{5}{2}{P_0}{V_0} \cr
& \Rightarrow {P_0}{V_0} = \dfrac{1}{3}R{T_0} \cr
& \Rightarrow \Delta Q = \dfrac{5}{2}\dfrac{{R{T_0}}}{3} \cr
& \therefore \Delta Q = \dfrac{{5R{T_0}}}{6} \cr} $
Hence I → Q, II → R, III → S, IV → U is correct
So option D is correct.
Note:
All the ideal gas laws we apply are valid only for the ideal gases. At low temperatures and high pressures there will be interactions between the molecules of the gases. Hence they deviate from the ideal gas behaviour. Usually gases behave as ideal gases at higher temperatures and lower pressures.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




