
The liquid air in fractional distillation method separates depending on:
(A) Melting point
(B) Density
(C) Volume
(D) Boiling point
Answer
516.6k+ views
Hint :Fractional distillation is a method which is used to separate mixtures into different components. In case of liquid air, it usually involves compression of liquid air, cooling, filtration and at last passing it to the distillation column to warm it up.
Complete Step By Step Answer:
Fractional distillation is the process of separation of a mixture into its component parts, or their fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of that mixture vaporizes.
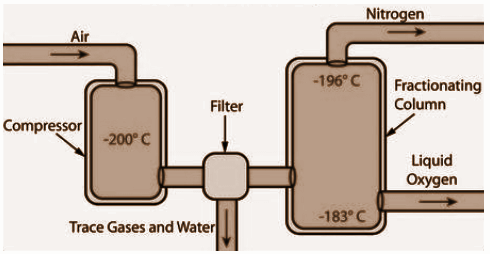
Fig.: Fractional distillation of liquid air
The liquid air in the fractional distillation method first gets compressed by increasing the pressure. Then, it is further cooled down by decreasing the temperature to again get the liquid air back. Sometimes, a filter might be used to remove the unnecessary gases, liquids or other particles from it. Now it is moved to a fractional distillation column to warm it up slowly. In this fractional distillation column, with the increase in temperature, gases start to get separated at different heights depending on their boiling points. The separated gases are obtained by the receiver ends from the fractional distillation column.
As we saw above liquid air gets separated depending on the boiling point of the gas.
Hence, option D is correct.
Note :
Whenever liquid air needs to be separated, fractional distillation is used. Focus on the steps involved in the separation of liquid air in fractional distillation. Making a diagram may help you to remember the steps correctly.
Complete Step By Step Answer:
Fractional distillation is the process of separation of a mixture into its component parts, or their fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of that mixture vaporizes.

Fig.: Fractional distillation of liquid air
The liquid air in the fractional distillation method first gets compressed by increasing the pressure. Then, it is further cooled down by decreasing the temperature to again get the liquid air back. Sometimes, a filter might be used to remove the unnecessary gases, liquids or other particles from it. Now it is moved to a fractional distillation column to warm it up slowly. In this fractional distillation column, with the increase in temperature, gases start to get separated at different heights depending on their boiling points. The separated gases are obtained by the receiver ends from the fractional distillation column.
As we saw above liquid air gets separated depending on the boiling point of the gas.
Hence, option D is correct.
Note :
Whenever liquid air needs to be separated, fractional distillation is used. Focus on the steps involved in the separation of liquid air in fractional distillation. Making a diagram may help you to remember the steps correctly.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




