
The linear structure is possesses by:
A) $\text{SnC}{{\text{l}}_{\text{2}}}$
B) $\text{NC}{{\text{O}}^{\text{-}}}$
C) $\text{NO}_{\text{2}}^{\text{+}}$
D) $\text{C}{{\text{S}}_{\text{2}}}$
Answer
568.2k+ views
Hint: The hybridisation tells about the geometry of the molecule. The hybridisation of a molecule can be determined by the steric number which can be calculated if we know the central atom of a molecule.
Complete answer:
So in the question it is given that we have to find those molecules which possess the linear structure.
For knowing a geometry of the we should know the hybridisation of the molecule.
So now let's discuss how we can predict the hybridisation of a molecule.
We can find the hybridisation using the steric number.
Steric number of a molecule is the number of other atoms attached to the central atom of a molecule or the number of lone pairs in the central atom.
\[\text{ }\!\!~\!\!\text{ Steric number= no}\text{.of }\!\! \sigma\!\! \text{ bonds of the central atom+ no}\text{.}\,\text{of}\,\text{lps in the central atom}\text{.}\]
The term lps in the equation refers to lone pairs of electrons in the central atom.
So now let's discuss each option.
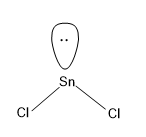
Option (A)- $\text{SnC}{{\text{l}}_{\text{2}}}$
Here Sn is the central atom and we know that Sn belongs to the group of C, which has a valency of 4,i.e. it can share 4 electrons for the bond formation.
Here in the molecule there are only two Cl atoms, which forms a sigma bond with the Sn and hence there will be two more electrons in the Sn which exists as the lone pair in Sn.
Now lets calculate the steric number for $\text{SnC}{{\text{l}}_{\text{2}}}$
There are two sigma bonds formed by Sn and a lone pair present in Sn.
Hence,\[Steric\text{ }Number\text{ }\left( S.N \right)=2+1=3\]
For steric number value 3, the hybridisation associated with it is $s{{p}^{2}}$, one s orbital and two p orbitals.
And the geometry related to $s{{p}^{2}}$ hybridisation is trigonal planar, but one of the axes of trigonal planar is occupied by the lone pair. Hence the geometry of $\text{SnC}{{\text{l}}_{\text{2}}}$ is bent shaped.
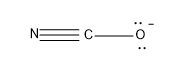
Option (B)- $\text{NC}{{\text{O}}^{\text{-}}}$
In the molecule the central atom is the C and it has a valency of 4 and can form four bonds with other atoms.
In this molecule as N is present it possesses a valency of 3 and forms one sigma bond and two pi bonds with C.The oxygen forms one sigma bond with C atom and it possesses a negative charge.
Now lets calculate the steric number,
\[Steric\text{ }Number\text{ }\left( S.N \right)=2\]
The hybridisation associated with steric number 2 is sp.
The geometry associated with sp hybridisation is linear.
Hence $\text{NC}{{\text{O}}^{\text{-}}}$ has a linear structure.
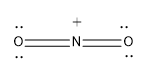
Option (C)- $\text{NO}_{\text{2}}^{\text{+}}$
Here nitrogen is the central atom and it shows an oxidation state of 3.
The nitrogen atom is forming the four bond with two O atoms two sigma bond and two pi bonds and it also possess a positive charge,as a electron deficiency is established in them
\[Steric\text{ }Number\text{ }\left( S.N \right)=2\]
As the steric number is two it possesses sp hybridisation and the geometry is linear.
Option (D)- $\text{C}{{\text{S}}_{\text{2}}}$
The C is the central atom; it can form four bonds with other atoms.
Here the C is forming Four bonds with the S atoms,two sigma bonds and two pi bonds and there is no lone pair as it obtains the octet configuration.
Now lets calculate the steric number,
\[Steric\text{ }Number\text{ }\left( S.N \right)=2\]
The hybridisation is sp and having a linear geometry.
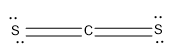
So the correct options are option (B), (C) and (D).
Note:
While calculating the steric number,confusion arises when multiple bonds are present,in a double bond-one bond is sigma and other is a sigma bond.For triple bond two bonds are pi bond and only one bond is sigma bond.
The lone pairs present in the central atom alter the geometry of the molecule to minimise the electronic repulsion.
Complete answer:
So in the question it is given that we have to find those molecules which possess the linear structure.
For knowing a geometry of the we should know the hybridisation of the molecule.
So now let's discuss how we can predict the hybridisation of a molecule.
We can find the hybridisation using the steric number.
Steric number of a molecule is the number of other atoms attached to the central atom of a molecule or the number of lone pairs in the central atom.
\[\text{ }\!\!~\!\!\text{ Steric number= no}\text{.of }\!\! \sigma\!\! \text{ bonds of the central atom+ no}\text{.}\,\text{of}\,\text{lps in the central atom}\text{.}\]
The term lps in the equation refers to lone pairs of electrons in the central atom.
So now let's discuss each option.
Option (A)- $\text{SnC}{{\text{l}}_{\text{2}}}$
Here Sn is the central atom and we know that Sn belongs to the group of C, which has a valency of 4,i.e. it can share 4 electrons for the bond formation.
Here in the molecule there are only two Cl atoms, which forms a sigma bond with the Sn and hence there will be two more electrons in the Sn which exists as the lone pair in Sn.
Now lets calculate the steric number for $\text{SnC}{{\text{l}}_{\text{2}}}$
There are two sigma bonds formed by Sn and a lone pair present in Sn.
Hence,\[Steric\text{ }Number\text{ }\left( S.N \right)=2+1=3\]
For steric number value 3, the hybridisation associated with it is $s{{p}^{2}}$, one s orbital and two p orbitals.
And the geometry related to $s{{p}^{2}}$ hybridisation is trigonal planar, but one of the axes of trigonal planar is occupied by the lone pair. Hence the geometry of $\text{SnC}{{\text{l}}_{\text{2}}}$ is bent shaped.

Option (B)- $\text{NC}{{\text{O}}^{\text{-}}}$
In the molecule the central atom is the C and it has a valency of 4 and can form four bonds with other atoms.
In this molecule as N is present it possesses a valency of 3 and forms one sigma bond and two pi bonds with C.The oxygen forms one sigma bond with C atom and it possesses a negative charge.
Now lets calculate the steric number,
\[Steric\text{ }Number\text{ }\left( S.N \right)=2\]
The hybridisation associated with steric number 2 is sp.
The geometry associated with sp hybridisation is linear.
Hence $\text{NC}{{\text{O}}^{\text{-}}}$ has a linear structure.

Option (C)- $\text{NO}_{\text{2}}^{\text{+}}$
Here nitrogen is the central atom and it shows an oxidation state of 3.
The nitrogen atom is forming the four bond with two O atoms two sigma bond and two pi bonds and it also possess a positive charge,as a electron deficiency is established in them
\[Steric\text{ }Number\text{ }\left( S.N \right)=2\]
As the steric number is two it possesses sp hybridisation and the geometry is linear.

Option (D)- $\text{C}{{\text{S}}_{\text{2}}}$
The C is the central atom; it can form four bonds with other atoms.
Here the C is forming Four bonds with the S atoms,two sigma bonds and two pi bonds and there is no lone pair as it obtains the octet configuration.
Now lets calculate the steric number,
\[Steric\text{ }Number\text{ }\left( S.N \right)=2\]
The hybridisation is sp and having a linear geometry.
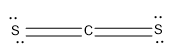
So the correct options are option (B), (C) and (D).
Note:
While calculating the steric number,confusion arises when multiple bonds are present,in a double bond-one bond is sigma and other is a sigma bond.For triple bond two bonds are pi bond and only one bond is sigma bond.
The lone pairs present in the central atom alter the geometry of the molecule to minimise the electronic repulsion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




