
The linear structure is assumed by:
This question has multiple correct options
A. $SnC{{l}_{2}}$
B. $NC{{O}^{-}}$
C. $C{{S}_{2}}$
D. $N{{O}_{2}}^{+}$
Answer
569.7k+ views
Hint: To tackle this question, one must really understand the Valence Shell Electron Pair Repulsion Theory (VSEPR) which helps in predicting the shape of the molecules. The Shape of the molecules is also dictated by the lone pair-lone pair and bond pair- bond pair repulsion. Therefore, if a lone pair is present on the molecule then it will change the bond angle of the molecule and hence, its shape.
Complete Solution :
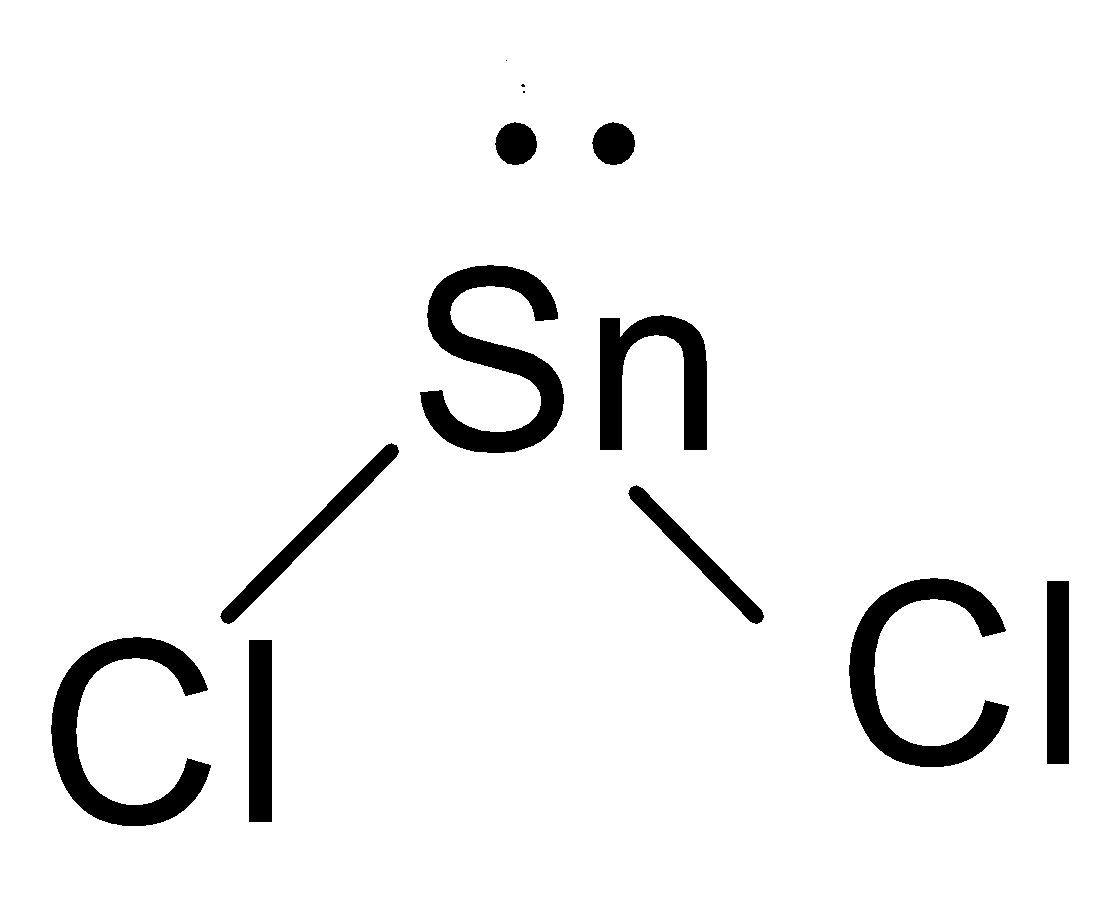
- First of all we will draw the shape of the molecules, In $SnC{{l}_{2}}$, the valence electron in Tin are 4 and in Chlorine there are 7, therefore, there will be two bonds created and one lone pair will be left on the Tin, which will repel the bond pair and hence, the shape of the molecule will be bent.
- In $NC{{O}^{-}}$, there are four valence electrons on Carbon so it can form 4 more bonds to complete its octet, and in Nitrogen, there are 5 electrons and Oxygen has 6 valence electrons. Therefore, Nitrogen forms three bonds with Carbon and Oxygen forms a single bond. Since, there are no lone pairs it will result in a linear shape.
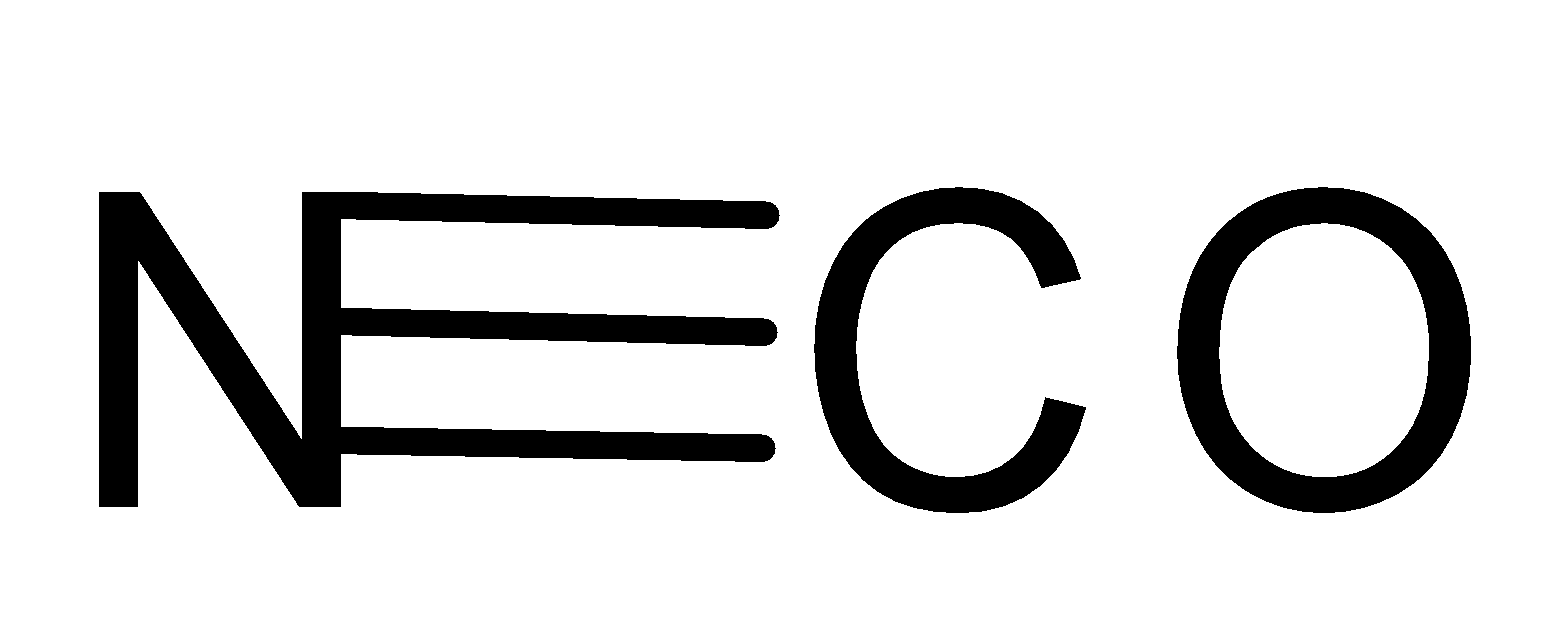
- In $C{{S}_{2}}$, again, Carbon has 4 valence electrons and Sulphur has 6 valence electrons and they both need 4 bonds to complete their octet, therefore , they form double bonds. Hence, this shape is also linear.
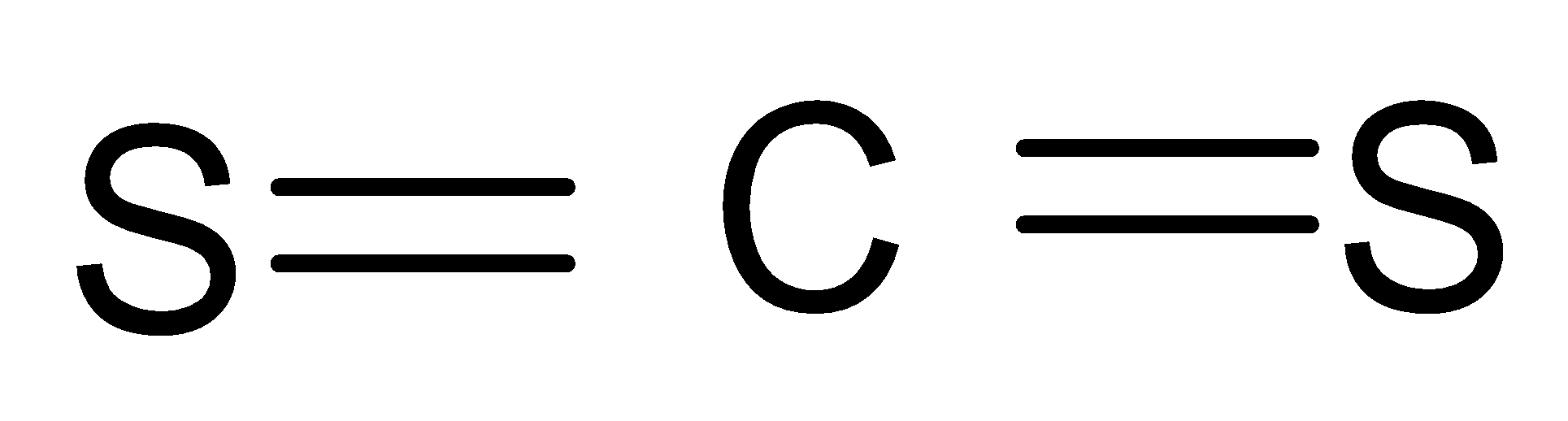
- In $N{{O}_{2}}^{+}$, Nitrogen has 5 valence electrons and Oxygen has 6 valence electrons and formal charge on the molecule is +1. Therefore, Nitrogen forms a double bond with both the Oxygen to complete them. This shape is also linear.
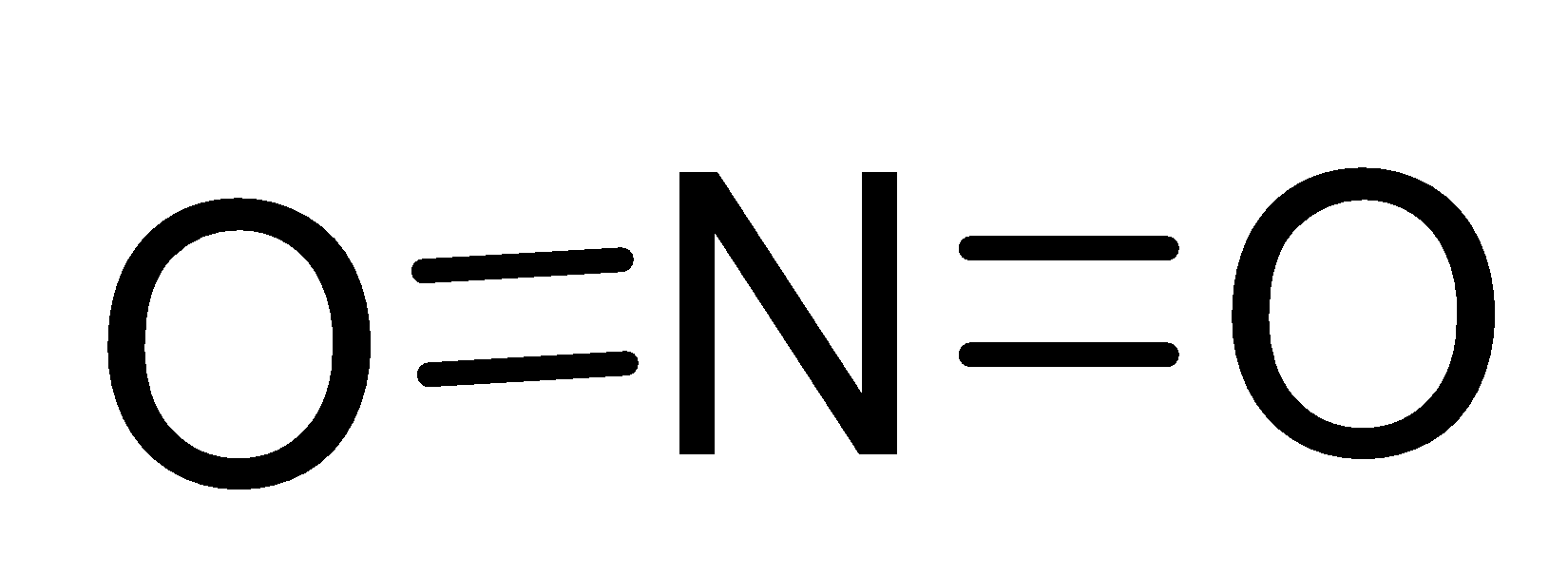
So, the correct answer is “Option B, C and D”.
Note: Every element wants to be stable and complete their octet, therefore they create bonds with other elements to share their electrons, this method is mostly valid to the elements belonging till the third period as it is a way. But, always calculate formal charge to understand the structure better.
Complete Solution :
- First of all we will draw the shape of the molecules, In $SnC{{l}_{2}}$, the valence electron in Tin are 4 and in Chlorine there are 7, therefore, there will be two bonds created and one lone pair will be left on the Tin, which will repel the bond pair and hence, the shape of the molecule will be bent.

- In $NC{{O}^{-}}$, there are four valence electrons on Carbon so it can form 4 more bonds to complete its octet, and in Nitrogen, there are 5 electrons and Oxygen has 6 valence electrons. Therefore, Nitrogen forms three bonds with Carbon and Oxygen forms a single bond. Since, there are no lone pairs it will result in a linear shape.
- In $C{{S}_{2}}$, again, Carbon has 4 valence electrons and Sulphur has 6 valence electrons and they both need 4 bonds to complete their octet, therefore , they form double bonds. Hence, this shape is also linear.
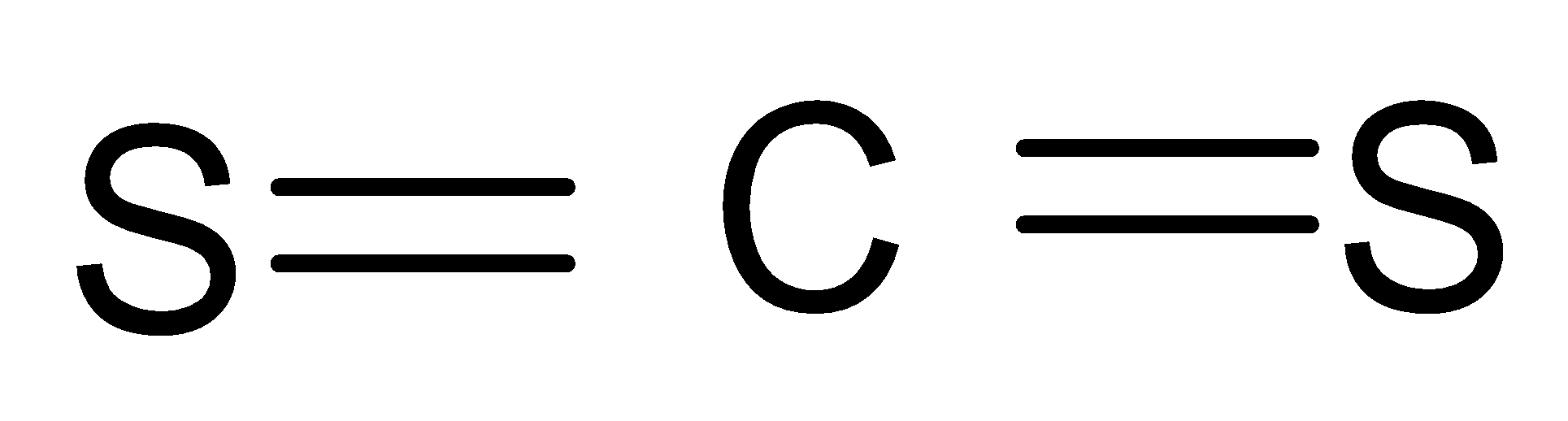
- In $N{{O}_{2}}^{+}$, Nitrogen has 5 valence electrons and Oxygen has 6 valence electrons and formal charge on the molecule is +1. Therefore, Nitrogen forms a double bond with both the Oxygen to complete them. This shape is also linear.
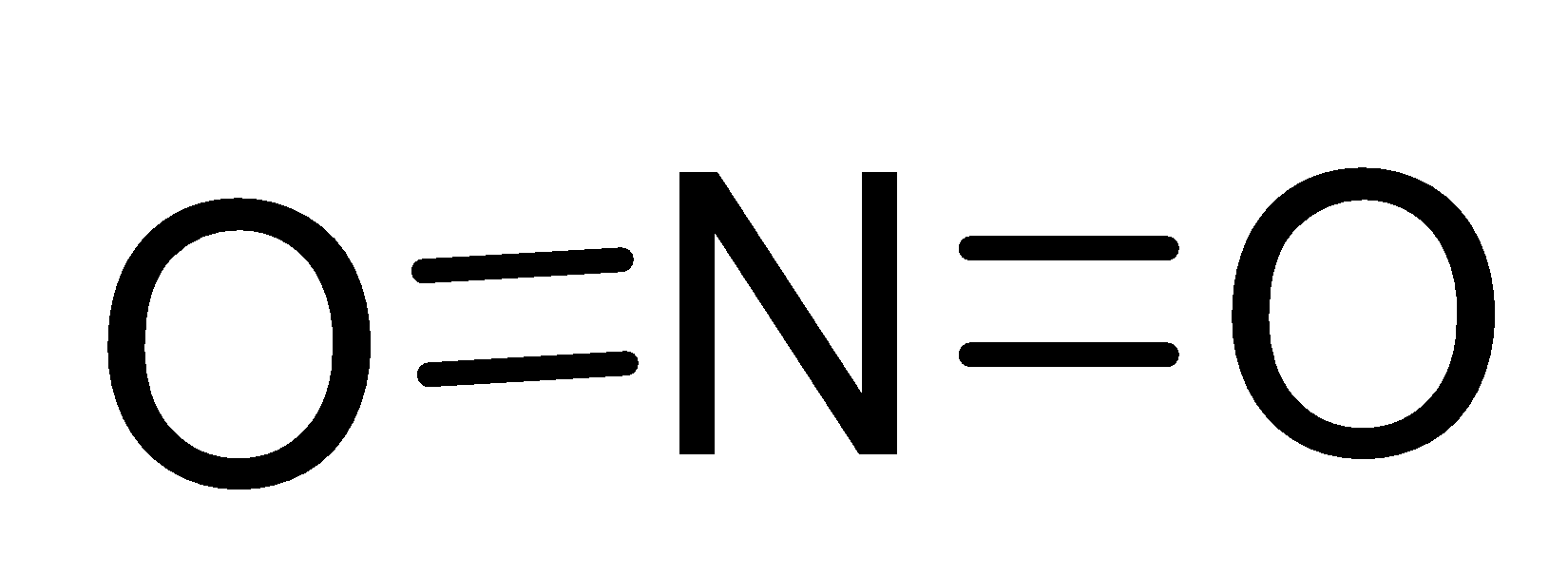
So, the correct answer is “Option B, C and D”.
Note: Every element wants to be stable and complete their octet, therefore they create bonds with other elements to share their electrons, this method is mostly valid to the elements belonging till the third period as it is a way. But, always calculate formal charge to understand the structure better.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




