
The ligand $N{{\left( C{{H}_{2}}\text{ C}{{\text{H}}_{2\text{ }}}N{{H}_{2}} \right)}_{3}}$ :
(A) Bidentate
(B) Tridentate
(C) Tetradentate
(D) Pentadentate
Answer
562.8k+ views
Hint: Check for the atoms having lone electron pairs, due to which they can form a bond with ligand. Then count the number of atoms present in the ligand with their total lone pairs. The number of lone pairs equals the bond formed with the ligand which will specify the ligand type.
Complete Step By Step Solution:
Ligand: It is any atom or molecule attached to a central atom, usually a metallic element, in a coordination compound. The ligands are almost always those which are capable of functioning as the electron pair donor in the electron pair bond which is the coordinate covalent bond.
Attachment of the ligand to the metal may be through a single atom that it is known as monodentate ligand, if through two atoms then didentate or bidentate, through three then tridentate, through four and five atoms then tetradentate and pentadentate respectively.
Lone Pair: It refers to the pair of valence electrons that are not shared with another atom in a covalent bond. These are also known as non-bonding pairs. These are as identified using Lewis structure.
We have, $\text{N}{{\left( \text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{2}}}\text{N}{{\text{H}}_{\text{2}}} \right)}_{\text{3}}}$ : In this ligand only nitrogen has one lone pair. It forms $3$ bonds and has one lone pair.
Example: In ammonia, $\text {N} {{\text{H}}_{3}}$
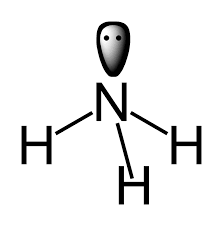
Here, in a given ligand we have a total $4$ nitrogen atom i.e. $4$ lone pair.
It means there are four bonds formed with the ligand. So it is a tetradentate ligand.
Additional Information:
Lewis structures also known as Lewis dot diagrams, these are diagrams that show the bonding between the atoms of the molecules and the lone pairs of electrons that may exist in the molecules. Lewis structure can be drawn for any covalently bonded molecule as well as coordination compounds.
A coordinate bond also known as Dative covalent Bond in which both electrons come from the same atom. A covalent bond is formed by two atoms sharing a pair of electrons. The atoms are held together because the electron pair is attracted by both of the nuclei. Covalent bond is the strongest among other bonds.
Note: Coordination compounds are molecules that pose one or multiple metal centers that are bound to ligands (atoms, ions or molecules that donate electrons to the metal).The concepts of ligands, lone pair and the coordinate chemistry should be clear. One should also learn about the Lewis structure.
Complete Step By Step Solution:
Ligand: It is any atom or molecule attached to a central atom, usually a metallic element, in a coordination compound. The ligands are almost always those which are capable of functioning as the electron pair donor in the electron pair bond which is the coordinate covalent bond.
Attachment of the ligand to the metal may be through a single atom that it is known as monodentate ligand, if through two atoms then didentate or bidentate, through three then tridentate, through four and five atoms then tetradentate and pentadentate respectively.
Lone Pair: It refers to the pair of valence electrons that are not shared with another atom in a covalent bond. These are also known as non-bonding pairs. These are as identified using Lewis structure.
We have, $\text{N}{{\left( \text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{2}}}\text{N}{{\text{H}}_{\text{2}}} \right)}_{\text{3}}}$ : In this ligand only nitrogen has one lone pair. It forms $3$ bonds and has one lone pair.
Example: In ammonia, $\text {N} {{\text{H}}_{3}}$

Here, in a given ligand we have a total $4$ nitrogen atom i.e. $4$ lone pair.
It means there are four bonds formed with the ligand. So it is a tetradentate ligand.
Additional Information:
Lewis structures also known as Lewis dot diagrams, these are diagrams that show the bonding between the atoms of the molecules and the lone pairs of electrons that may exist in the molecules. Lewis structure can be drawn for any covalently bonded molecule as well as coordination compounds.
A coordinate bond also known as Dative covalent Bond in which both electrons come from the same atom. A covalent bond is formed by two atoms sharing a pair of electrons. The atoms are held together because the electron pair is attracted by both of the nuclei. Covalent bond is the strongest among other bonds.
Note: Coordination compounds are molecules that pose one or multiple metal centers that are bound to ligands (atoms, ions or molecules that donate electrons to the metal).The concepts of ligands, lone pair and the coordinate chemistry should be clear. One should also learn about the Lewis structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




