
The laws violated in the given electronic configuration of oxygen atoms are
Aufbau rule
Pauli exclusion principle
Hund’s rule
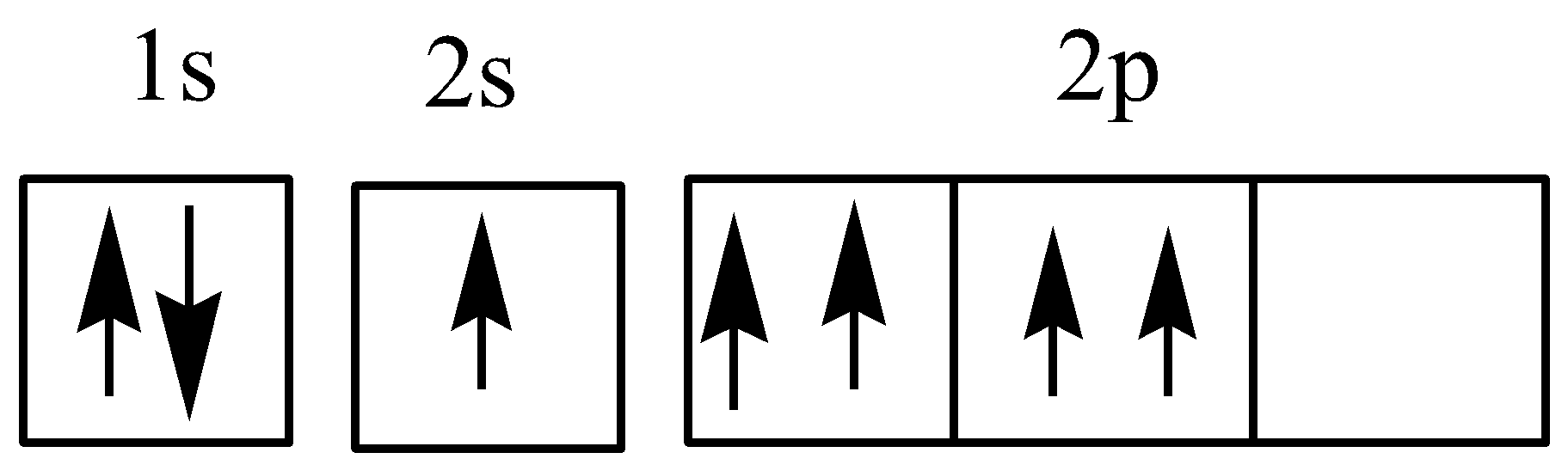
A.a and b
B.b and c
C.a and c
D.a, b and c

Answer
581.4k+ views
Hint: The electronic configuration of Oxygen is $1{s^2}2{s^2}2{p^4}$ In Aufbau rule, lower energy orbitals fill before the higher one. In Pauli exclusion principle, no two electrons can be identified by the same sets of quantum numbers and in Hund’s rule, one electron goes first in all orbitals before pairing up.
Complete step by step answer:
While writing the electronic configuration for oxygen, the first two electrons will go in 1s orbital, next two in 2s and remaining four in 2p orbital.
According to the Aufbau principle, it states that electrons are filled into atomic orbitals in the increasing order of orbital energy level. The electrons first occupy those orbitals whose energy is the lowest. For example, the orbitals in \[n = 1\] will before orbital in \[n = 2\] . But in a given electronic configuration, we see that there is only one electron in 2s orbital but two in 2p.
The Pauli exclusion principle explains that, it is important to note that each orbital can hold a maximum of two electrons and no two electrons have the same four electronic quantum numbers.
According to Hund’s rule, every orbital in a given subshell must be singly occupied before it is doubly occupied in an orbital. The electrons first fill all the orbitals with the same energy.
As per the Hund’s rule, the electronic configuration of oxygen atom should be:
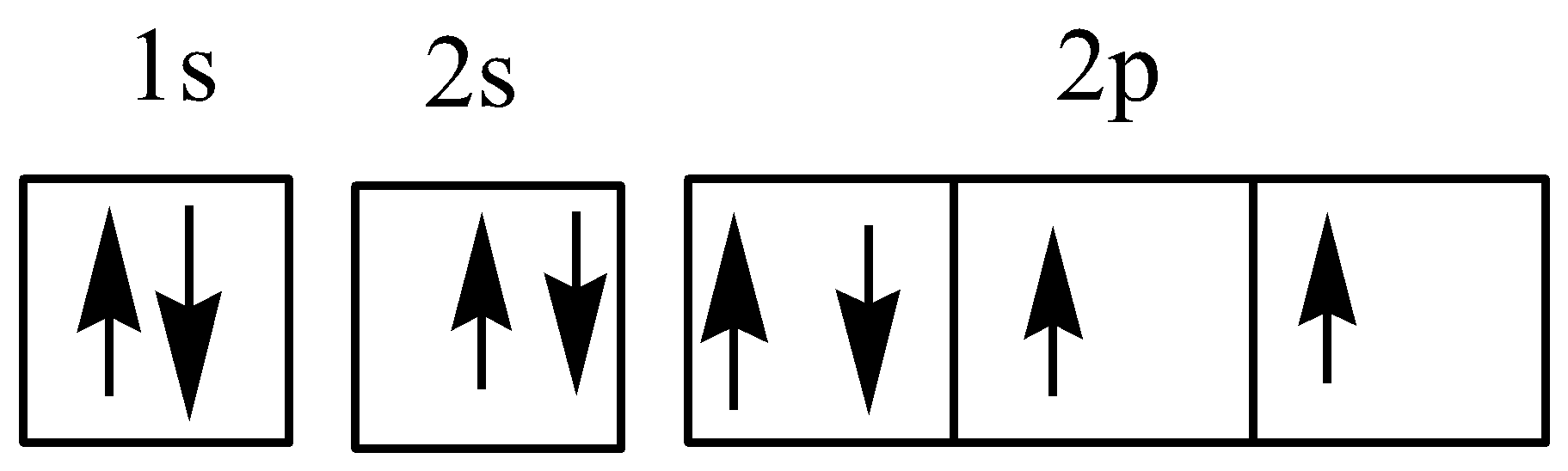
Hence, the given electronic configuration violates the Aufbau rule, Pauli exclusion principle as well as the Hund’s rule.
Therefore, the correct answer is option (D).
Note: The order in which the orbitals are filled with electrons is: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on.
Complete step by step answer:
While writing the electronic configuration for oxygen, the first two electrons will go in 1s orbital, next two in 2s and remaining four in 2p orbital.
According to the Aufbau principle, it states that electrons are filled into atomic orbitals in the increasing order of orbital energy level. The electrons first occupy those orbitals whose energy is the lowest. For example, the orbitals in \[n = 1\] will before orbital in \[n = 2\] . But in a given electronic configuration, we see that there is only one electron in 2s orbital but two in 2p.
The Pauli exclusion principle explains that, it is important to note that each orbital can hold a maximum of two electrons and no two electrons have the same four electronic quantum numbers.
According to Hund’s rule, every orbital in a given subshell must be singly occupied before it is doubly occupied in an orbital. The electrons first fill all the orbitals with the same energy.
As per the Hund’s rule, the electronic configuration of oxygen atom should be:

Hence, the given electronic configuration violates the Aufbau rule, Pauli exclusion principle as well as the Hund’s rule.
Therefore, the correct answer is option (D).
Note: The order in which the orbitals are filled with electrons is: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




