
The IUPAC name of the following group
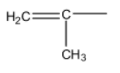
A. Isopropenyl
B. 1-Methylethenyl
C. 2-Methylethylnyl
D. None of the above

Answer
578.4k+ views
Hint: According to IUPAC (International Union of Pure and Applied Chemistry), whenever we are going to write the IUPAC name of a compound, we have to give numbering first to functional groups or highly substituted carbon. Means lower numbering should be a functional group or highly substituted carbon present in the molecule or compound.
Complete step by step solution:
- In the question it is given to write the IUPAC name of the given compound.
- There are some rules and regulations to write the IUPAC names for organic compounds of hydrocarbons.
Rules for writing IUPAC name for Hydrocarbons:-
- Initially we have to select the longest carbon chain in the given hydrocarbon.
- Next we have to give numbers to the longest chain.
- At the time of numbering the longest chain is numbered from starting or from a terminal carbon. Numbering has to be done in such a way that the lowest number should be given to the side chain or a functional group which is present.
- Next writing names to the branched or saturated hydrocarbons followed by the name of the parent chain.
- Now we have to apply all these rules to the given molecule and it is as follows.
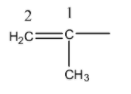
- There is a presence of unsaturation at carbon-1.
- The longest chain contains two carbons and one substituent present on carbon-1 that is methyl.
- Therefore the IUPAC name of the given group is 1-Methylethenyl.
So, the correct option is B.
Note: If a double bond is present in the hydrocarbon ‘ene’ should come at the end of the IUPAC name of the compound. If a triple bond is present in the hydrocarbon, ‘yne’ should come at the end of the IUPAC name of the compound.
Complete step by step solution:
- In the question it is given to write the IUPAC name of the given compound.
- There are some rules and regulations to write the IUPAC names for organic compounds of hydrocarbons.
Rules for writing IUPAC name for Hydrocarbons:-
- Initially we have to select the longest carbon chain in the given hydrocarbon.
- Next we have to give numbers to the longest chain.
- At the time of numbering the longest chain is numbered from starting or from a terminal carbon. Numbering has to be done in such a way that the lowest number should be given to the side chain or a functional group which is present.
- Next writing names to the branched or saturated hydrocarbons followed by the name of the parent chain.
- Now we have to apply all these rules to the given molecule and it is as follows.

- There is a presence of unsaturation at carbon-1.
- The longest chain contains two carbons and one substituent present on carbon-1 that is methyl.
- Therefore the IUPAC name of the given group is 1-Methylethenyl.
So, the correct option is B.
Note: If a double bond is present in the hydrocarbon ‘ene’ should come at the end of the IUPAC name of the compound. If a triple bond is present in the hydrocarbon, ‘yne’ should come at the end of the IUPAC name of the compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




