
The IUPAC name of the following compound is 4-(ethoxycarbonyl) benzoic acid.
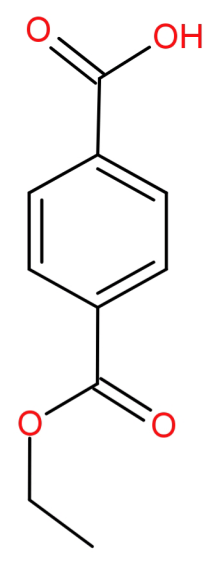
(A)True
(B)False

Answer
600k+ views
Hint: First choose the longest carbon chain to be the parent chain and then see which of the functional groups is of highest priority. Start numbering the chain from the position of most prior functional groups towards the substituent.
Complete answer:
-The first step in IUPAC nomenclature is identifying the longest carbon chain.
Here the longest chain is the 6 carbon cyclic structure benzene.
Also, in the given structure there are 2 functional groups. They are carboxylic acid and ester group out of which carboxylic acid is of highest priority and thus the major functional group.
So, the parent chain will be the benzene with carboxylic acid which is called benzoic acid.
-Now we will look at the substituent group. In this there is an ethyl group attached to the oxygen atom of the carbonyl group and so it will be known as ethoxycarbonyl.
This ethoxycarbonyl group is attached at 4th position while numbering from position of carboxylic acid. The numbering will be done as follows:
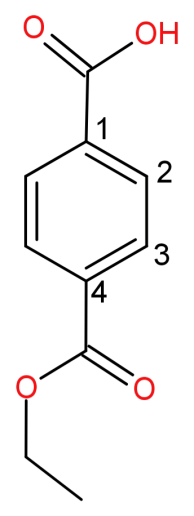
So, the IUPAC name of this structure will be 4-(ethoxycarbonyl) benzoic acid. It has a molecular formula of: ${C_{10}}{H_{10}}{O_4}$.
Hence the correct option is: (A) True.
Note:
The priority order of the functional groups is: carboxylic acid > sulfonic acid > ester > acid halide > amide > nitrile > aldehyde > ketone > alcohol > thiol > amine. If any compound has two or more functional groups the numbering is always done on the basis of the priority of the functional groups.
Complete answer:
-The first step in IUPAC nomenclature is identifying the longest carbon chain.
Here the longest chain is the 6 carbon cyclic structure benzene.
Also, in the given structure there are 2 functional groups. They are carboxylic acid and ester group out of which carboxylic acid is of highest priority and thus the major functional group.
So, the parent chain will be the benzene with carboxylic acid which is called benzoic acid.
-Now we will look at the substituent group. In this there is an ethyl group attached to the oxygen atom of the carbonyl group and so it will be known as ethoxycarbonyl.
This ethoxycarbonyl group is attached at 4th position while numbering from position of carboxylic acid. The numbering will be done as follows:

So, the IUPAC name of this structure will be 4-(ethoxycarbonyl) benzoic acid. It has a molecular formula of: ${C_{10}}{H_{10}}{O_4}$.
Hence the correct option is: (A) True.
Note:
The priority order of the functional groups is: carboxylic acid > sulfonic acid > ester > acid halide > amide > nitrile > aldehyde > ketone > alcohol > thiol > amine. If any compound has two or more functional groups the numbering is always done on the basis of the priority of the functional groups.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




