
The IUPAC name of the following compound is:
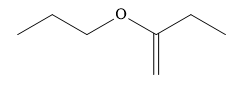

Answer
579.9k+ views
Hint: You can name ethers as alkoxy alkanes. You can indicate the presence of carbon-carbon double bond by using the suffix ‘ene’.
Complete step by step answer:
Alkoxy alkanes represents ethers. Ethers are the compounds having general formula \[{\text{R}} - {\text{O}} - {\text{R'}}\]. The functional group present is an ether functional group, represented as \[ - {\text{O}} - \]. Two alkyl groups are joined to ether functional group. While naming ethers, the alkyl group containing fewer carbon atoms is named as alkoxy group.
Alkenes are unsaturated hydrocarbons. They contain \[{\text{C}} = {\text{C}}\] double bonds.
Write the structure of the given compound:
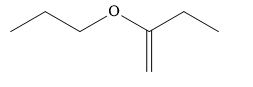
It contains an ether functional group and a \[{\text{C}} = {\text{C}}\] double bond.
In the ether, two alkyl groups are present. One alkyl group is a straight chain containing 3 carbon atoms. It is named as a prefix of propoxy. The other substituent is also a straight chain containing 4 carbon atoms and a \[{\text{C}} = {\text{C}}\] double bond. Butane is a saturated hydrocarbon containing 4 carbon atoms. Butene is an unsaturated hydrocarbon containing 4 carbon atoms and a \[{\text{C}} = {\text{C}}\] double bond.
In the given compound, the propoxy substituent is present on the second carbon atom and \[{\text{C}} = {\text{C}}\] double bond present between the first and second carbon atom. The numbering of carbon chain is started from the end which gives lowest possible locants to double bonded carbon atoms.
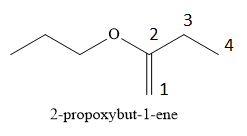
The IUPAC name of the following compound is 2-propoxybut-1-ene.
Note: You can classify ethers into symmetrical ethers and unsymmetrical ethers. In symmetrical ethers, both functional groups are the same. In unsymmetrical ethers, two functional groups are different. An example of symmetrical ether is diethyl ether in which two alkyl groups are ethyl groups. An example of unsymmetrical ether is ethyl methyl ether in which two alkyl groups are ethyl group and methyl group.
Complete step by step answer:
Alkoxy alkanes represents ethers. Ethers are the compounds having general formula \[{\text{R}} - {\text{O}} - {\text{R'}}\]. The functional group present is an ether functional group, represented as \[ - {\text{O}} - \]. Two alkyl groups are joined to ether functional group. While naming ethers, the alkyl group containing fewer carbon atoms is named as alkoxy group.
Alkenes are unsaturated hydrocarbons. They contain \[{\text{C}} = {\text{C}}\] double bonds.
Write the structure of the given compound:

It contains an ether functional group and a \[{\text{C}} = {\text{C}}\] double bond.
In the ether, two alkyl groups are present. One alkyl group is a straight chain containing 3 carbon atoms. It is named as a prefix of propoxy. The other substituent is also a straight chain containing 4 carbon atoms and a \[{\text{C}} = {\text{C}}\] double bond. Butane is a saturated hydrocarbon containing 4 carbon atoms. Butene is an unsaturated hydrocarbon containing 4 carbon atoms and a \[{\text{C}} = {\text{C}}\] double bond.
In the given compound, the propoxy substituent is present on the second carbon atom and \[{\text{C}} = {\text{C}}\] double bond present between the first and second carbon atom. The numbering of carbon chain is started from the end which gives lowest possible locants to double bonded carbon atoms.

The IUPAC name of the following compound is 2-propoxybut-1-ene.
Note: You can classify ethers into symmetrical ethers and unsymmetrical ethers. In symmetrical ethers, both functional groups are the same. In unsymmetrical ethers, two functional groups are different. An example of symmetrical ether is diethyl ether in which two alkyl groups are ethyl groups. An example of unsymmetrical ether is ethyl methyl ether in which two alkyl groups are ethyl group and methyl group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




