
The IUPAC name of the compound is:
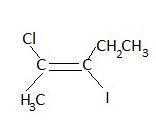
(a) trans-2-chloro-3-iodo-2-pentene
(b) cis-2-chloro-3-iodo-2-pentane
(c) trans-3-iodo-4-chloro-3-pentane
(d) cis-3-iodo-4-chloro-3-pentane

Answer
581.7k+ views
Hint: IUPAC has certain set rules for the naming of chemical compounds. This ensures that no two compounds will have the same nomenclature. We first need to find the long chain, assign numbers to the carbons, taking into consideration the substituents and the double bond. For cis and trans, we need to apply CIP rules.
Complete step by step answer:
Given: The structure of a compound (a substituted alkene) is given.
Steps:
To assign a name to the compound given above, the following steps should be done:
1. First, we select the longest hydrocarbon chain. Here it is a five carbon chain with a double bond. So, it’s a pentene (-ene suffix for alkenes).
2. Then, based on the fact that the substituents and double bonds need to be assigned as low a number as possible, when we assign the numbers for C atoms, we try to do it from the right hand side first. Then, it is assigned 3 for Iodine, 3 for double bond and 4 for Chlorine.
3. Assigning from the left hand side, we get the number 2, 2 and 3 for Chlorine, double bond and Iodine respectively.
4. Thus, we assign numbering from the left hand side as the double bond gets the lowest number along with the other substituents. This is known as the lowest set of locants rules. The numbers represent locants/location of a group on the carbon chain. The locant set (2,2,3) is the lowest set that can be assigned when numbered from the left hand side.
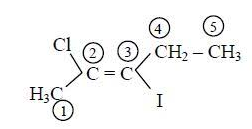
5. Next, to assign cis or trans to the compound, we need to look at the upper and lower groups assigned to the double bond.
6. On the right hand side, we have an ethyl group on the upper side and an iodine group to the lower side. Using the CIP rule, we can assign priorities to the two groups. We need to look at the atomic number of the directly attached atom first. If it's the same, then we consider the atomic weight. Here, Iodine is assigned 1 and Carbon 2.
7. Similarly, for the left hand side, chlorine is assigned 1 and methyl group 2.
8. The number (priority) 1 is downward while the other is upward. Similarly, the number 2 is downward on one side and oppositely placed on the other. Thus, the compound is a trans compound.
9. Thus, considering all these observations, the nomenclature of the compound comes to be:
Trans-2-chloro-3-iodo-2-pentene.
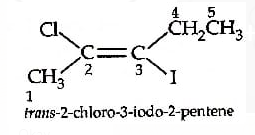
Thus, the correct option is (a).
Note:
While naming the compound, we write the different substituents alphabetically, so chloro followed by iodo. Also, as seen above the double bond must get the lowest number possible.IUPAC and CIP rules should be thoroughly studied by the students.
Complete step by step answer:
Given: The structure of a compound (a substituted alkene) is given.
Steps:
To assign a name to the compound given above, the following steps should be done:
1. First, we select the longest hydrocarbon chain. Here it is a five carbon chain with a double bond. So, it’s a pentene (-ene suffix for alkenes).
2. Then, based on the fact that the substituents and double bonds need to be assigned as low a number as possible, when we assign the numbers for C atoms, we try to do it from the right hand side first. Then, it is assigned 3 for Iodine, 3 for double bond and 4 for Chlorine.
3. Assigning from the left hand side, we get the number 2, 2 and 3 for Chlorine, double bond and Iodine respectively.
4. Thus, we assign numbering from the left hand side as the double bond gets the lowest number along with the other substituents. This is known as the lowest set of locants rules. The numbers represent locants/location of a group on the carbon chain. The locant set (2,2,3) is the lowest set that can be assigned when numbered from the left hand side.

5. Next, to assign cis or trans to the compound, we need to look at the upper and lower groups assigned to the double bond.
6. On the right hand side, we have an ethyl group on the upper side and an iodine group to the lower side. Using the CIP rule, we can assign priorities to the two groups. We need to look at the atomic number of the directly attached atom first. If it's the same, then we consider the atomic weight. Here, Iodine is assigned 1 and Carbon 2.
7. Similarly, for the left hand side, chlorine is assigned 1 and methyl group 2.
8. The number (priority) 1 is downward while the other is upward. Similarly, the number 2 is downward on one side and oppositely placed on the other. Thus, the compound is a trans compound.
9. Thus, considering all these observations, the nomenclature of the compound comes to be:
Trans-2-chloro-3-iodo-2-pentene.

Thus, the correct option is (a).
Note:
While naming the compound, we write the different substituents alphabetically, so chloro followed by iodo. Also, as seen above the double bond must get the lowest number possible.IUPAC and CIP rules should be thoroughly studied by the students.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




