
The IUPAC name of the compound is:
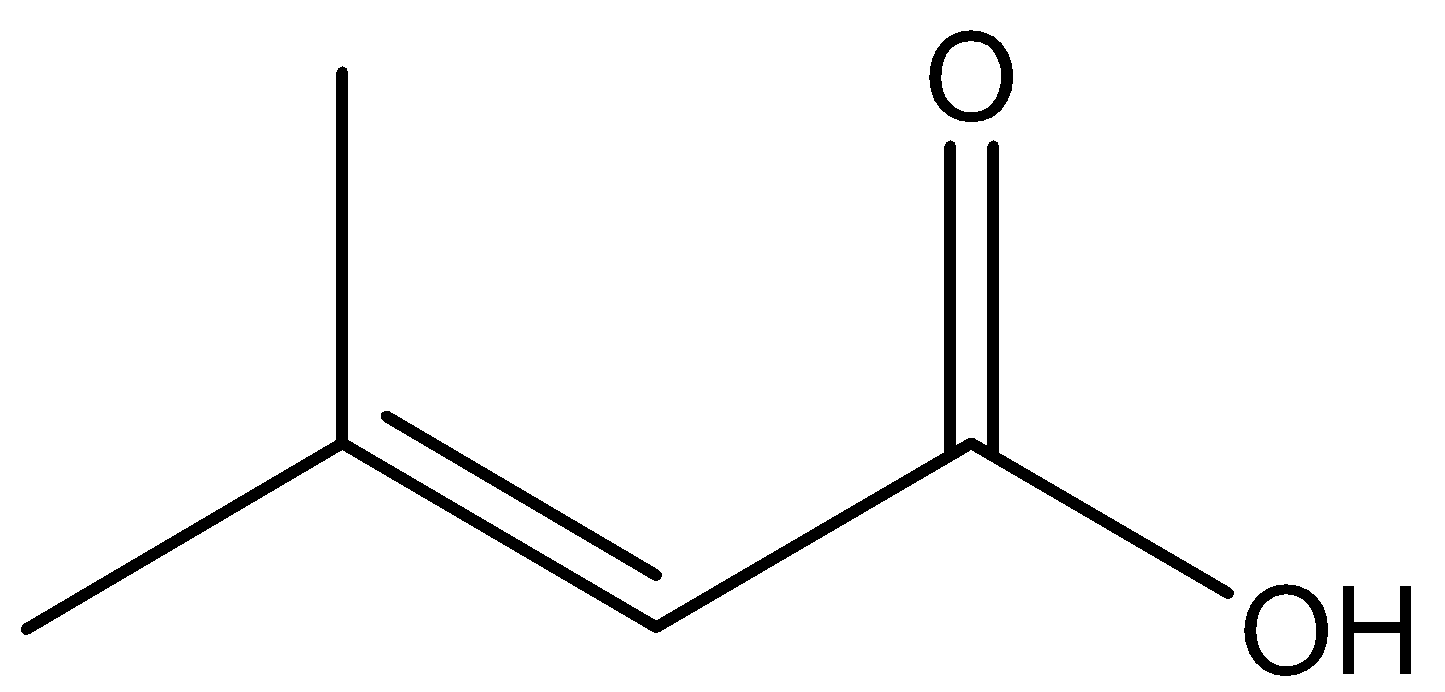
(A) $ 2 $ - methyl - $ 2 $ - butenoic acid
(B) $ 3 $ - methyl - $ 3 $ - butenoic acid
(C) $ 3 $ -methyl but- $ 2 $ - enoic acid
(D) $ 2 $ - methyl - $ 3 $ - butenoic acid

Answer
556.2k+ views
Hint: We will use the guidelines laid by the IUPAC (International Union of Pure and Applied Chemistry) to name the following chemical compound. IUPAC is an international chemistry union to which all the countries have agreed so that there are no two names for the same chemical compound. The longest chain will be the base compound with the substituents written as prefixes.
Complete step by step solution
In the above chemical compound, we can observe that there is a $ COOH $ group as well as a double bond present. But $ COOH $ is given more priority as compared to double bonds. So, we will start with $ COOH $ as the first carbon atom and start identifying the longest carbon chain.
Then after identifying the longest carbon atom chain, we will start numbering the longest chain. The longest carbon chain consists of $ 4 $ carbon atoms.
On the third carbon atom from the right-hand side, a $ C{{H}_{3}} $ group is attached, so we number it as $ 3 $ -methyl.
Also, we can observe that there is a double bond attached to the second carbon atom, and we have a total $ 4 $ carbon atom in the chain, so we will name it as butane. But there is a double bond in the second position. So, its name will be rewritten as but- $ 2 $ - ene.
Also, in the first position, there is the $ COOH $ group. $ COOH $ is an acid group, so the name will be but- $ 2 $ -enoic acid.
Hence, the IUPAC name of the chemical compound is $ 3 $ -methyl but- $ 2 $ - enoic acid.
The correct option is (C).
Note
The need for such a systematic approach arose because of the sheer quantity of new organic compound discoveries that made the trivial nomenclature of organic compounds extremely inconvenient. However, chemists do not always follow the IUPAC nomenclature guidelines because some compounds, as per the IUPAC nomenclature guidelines, have very long and extremely tedious names. More trivial names are assigned to these compounds.
Complete step by step solution
In the above chemical compound, we can observe that there is a $ COOH $ group as well as a double bond present. But $ COOH $ is given more priority as compared to double bonds. So, we will start with $ COOH $ as the first carbon atom and start identifying the longest carbon chain.
Then after identifying the longest carbon atom chain, we will start numbering the longest chain. The longest carbon chain consists of $ 4 $ carbon atoms.
On the third carbon atom from the right-hand side, a $ C{{H}_{3}} $ group is attached, so we number it as $ 3 $ -methyl.
Also, we can observe that there is a double bond attached to the second carbon atom, and we have a total $ 4 $ carbon atom in the chain, so we will name it as butane. But there is a double bond in the second position. So, its name will be rewritten as but- $ 2 $ - ene.
Also, in the first position, there is the $ COOH $ group. $ COOH $ is an acid group, so the name will be but- $ 2 $ -enoic acid.
Hence, the IUPAC name of the chemical compound is $ 3 $ -methyl but- $ 2 $ - enoic acid.
The correct option is (C).
Note
The need for such a systematic approach arose because of the sheer quantity of new organic compound discoveries that made the trivial nomenclature of organic compounds extremely inconvenient. However, chemists do not always follow the IUPAC nomenclature guidelines because some compounds, as per the IUPAC nomenclature guidelines, have very long and extremely tedious names. More trivial names are assigned to these compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




