
The IUPAC name of the compound
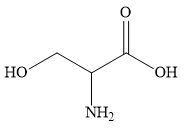
A ) 2-amino-3-hydroxypropanoic acid
B ) 1-hydroxy-2-amino propan-3-oic acid
C ) 1-amino-2-hydroxy propanoic acid
D ) 3-hydroxy-2-amino propanoic acid

Answer
581.7k+ views
Hint: Determine the number of carbon atoms in the parent carbon chain. Identify different substituents and the principal functional group. Number the chain from the end containing the carboxylic functional group. Use alphabetical order of the substituents.
Complete step by step answer:
The longest continuous chain contains 3 carbon atoms. Hence, the parent carbon atom is propane.
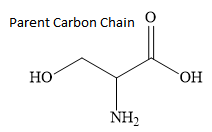
Three different functional groups are present. These are carboxylic group, amino group and hydroxyl group.
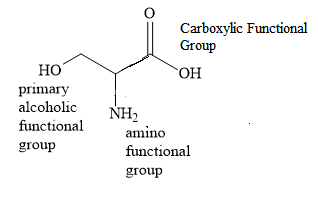
Carboxylic group gets preference over amino and hydroxyl groups. Hence, the suffix in the IUPAC name is propanoic acid. In the name of the corresponding alkane, propane, the suffix ‘ane’ is replaced with the suffix ‘oic acid’ to obtain the name propanoic acid.
The numbering of the chain is started from the end containing carboxylic group. The carbon atom of the carboxylic acid gets number 1, the carbon atom of amino group gets number 2 and the carbon atom of hydroxyl group gets number 3.
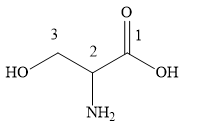
Two substituents amino and hydroxyl are named in the alphabetical order as prefixes amino and hydroxyl.
The IUPAC name of the given compound is 2-amino-3-hydroxypropanoic acid.
Hence, the option A ) is the correct answer.
Note: The name 1-hydroxy-2-amino propan-3-oic acid is incorrect as the numbering of the chain is not started from the end containing hydroxyl group.
The name 1-amino-2-hydroxy propanoic acid is incorrect as the numbering of the chain is not started from the carbon containing amino group.
The name 3-hydroxy-2-amino propanoic acid is incorrect as the alphabetical order of substituents is not followed.
Complete step by step answer:
The longest continuous chain contains 3 carbon atoms. Hence, the parent carbon atom is propane.

Three different functional groups are present. These are carboxylic group, amino group and hydroxyl group.

Carboxylic group gets preference over amino and hydroxyl groups. Hence, the suffix in the IUPAC name is propanoic acid. In the name of the corresponding alkane, propane, the suffix ‘ane’ is replaced with the suffix ‘oic acid’ to obtain the name propanoic acid.
The numbering of the chain is started from the end containing carboxylic group. The carbon atom of the carboxylic acid gets number 1, the carbon atom of amino group gets number 2 and the carbon atom of hydroxyl group gets number 3.

Two substituents amino and hydroxyl are named in the alphabetical order as prefixes amino and hydroxyl.
The IUPAC name of the given compound is 2-amino-3-hydroxypropanoic acid.
Hence, the option A ) is the correct answer.
Note: The name 1-hydroxy-2-amino propan-3-oic acid is incorrect as the numbering of the chain is not started from the end containing hydroxyl group.
The name 1-amino-2-hydroxy propanoic acid is incorrect as the numbering of the chain is not started from the carbon containing amino group.
The name 3-hydroxy-2-amino propanoic acid is incorrect as the alphabetical order of substituents is not followed.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




